Page 222 - Feline Cardiology
P. 222
Chapter 18: Arrhythmias and Other Electrocardiographic Abnormalities 227
petence, recurrence of congestive heart failure, or other cats. Nevertheless, since AF in cats is almost invariably
signs of beta-blocker excess), diltiazem HCl (7.5 mg PO associated with atrial enlargement, anticoagulation is
q 8h; avoid sustained-release diltiazem [Dilacor-XL, Cardizem- warranted on the basis of atrial enlargement alone (see
CD], where pharmacokinetics are unpredictable in Chapter 20).
the cat (Johnson et al. 1996; Wall et al. 2005) and Complications of AF and its treatment may include
gastrointestinal/hepatobiliary side effects occur in up to medication intolerance (see description, above) and
20% of cats), or digoxin (0.03125 [1/4 of a 0.125 mg progression of underlying heart disease leading to con-
tablet] PO q 48 h; never in hypertrophic cardiomyopa- gestive heart failure, thromboembolism, and/or sudden
thy; always with careful monitoring for inappetence, cardiac death. The occurrence of such complications is
lethargy, vomiting, or diarrhea as signs of digoxin highly variable between cats, and the prognosis for AF
intolerance/toxicosis). The therapeutic target is typically in the cat covers a wide range. The median survival in a
to achieve an exam room heart rate of 240 beats/minute retrospective study of 24 cats with AF was 165 days, with
or less. Since this is often already the case for many cats more than half living >6 months (6–12 months: 21%; 1
with AF before treatment, the presence of AF in the cat year or more: 33%) (Côté et al. 2004). Overall, the sur-
does not automatically require treatment. Without sub- vival of cats with AF is longer than that of cats with
stantiation, cardiologists and clinicians may be more aortic thromboembolism, shorter than that of cats with
inclined to prescribe treatment for asymptomatic struc- asymptomatic/compensated heart disease, and similar
tural heart disease (e.g., HCM) if AF is also present, to that of cats with congestive heart failure (Atkins et al. Arrhythmias
even at a nonelevated rate, with the intent of using a 2001).
beta blocker to help both with the cardiomyopathic
process and with heart rate limitation related to AF. Premature Ventricular Complexes
Holter monitoring, commonly used in dogs and humans Perhaps the most widely recognized, and misinterpreted,
with AF to better appreciate heart rate through a 24- ECG abnormality is the premature ventricular complex
hour period (and away from the hospital) is currently (PVC; also called ventricular premature contraction,
little used in feline cardiology for this purpose; ethical depolarization, or extrasystole [VPC/VPD/VE]). The
concerns about the burden of the monitors’ weight premature, abnormal-appearing (often wide, bizarre in
being carried by cats with heart disease, monitor avail- morphology) QRS complex and T wave of a PVC are the
ability, and additional expense, are important limita- electrocardiographic expression of spontaneous electri-
tions. The result is likely an underappreciation of the cal activity originating in the ventricles and serving no
need (or lack thereof) for treatment of AF. Atrial fibril- useful purpose (Figures 18.12, 18.13).
lation is associated with thromboembolic disease in The mechanisms by which premature ventricular
human patients, a result of the loss of atrial contraction complexes occur are likely the same, at the ventricular
and resultant sluggish flow of blood in fibrillating atria, level, as those at the atrial level that cause PACs (above):
but such an association has not been documented in abnormal automaticity, microreentry, and delayed
1
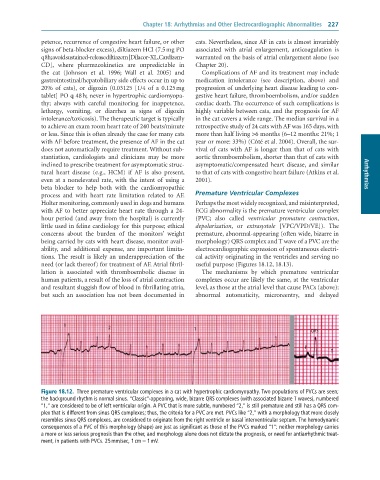
2 1 QRS
P
T
Figure 18.12. Three premature ventricular complexes in a cat with hypertrophic cardiomyopathy. Two populations of PVCs are seen;
the background rhythm is normal sinus. “Classic”-appearing, wide, bizarre QRS complexes (with associated bizarre T waves), numbered
“1,” are considered to be of left ventricular origin. A PVC that is more subtle, numbered “2,” is still premature and still has a QRS com-
plex that is different from sinus QRS complexes; thus, the criteria for a PVC are met. PVCs like “2,” with a morphology that more closely
resembles sinus QRS complexes, are considered to originate from the right ventricle or basal interventricular septum. The hemodynamic
consequences of a PVC of this morphology (shape) are just as significant as those of the PVCs marked “1”; neither morphology carries
a more or less serious prognosis than the other, and morphology alone does not dictate the prognosis, or need for antiarrhythmic treat-
ment, in patients with PVCs. 25 mm/sec, 1 cm = 1 mV.