Page 154 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 154
Regulatory Aspects for the Drugs and Chemicals Used in Food-Producing Animals Chapter | 7 121
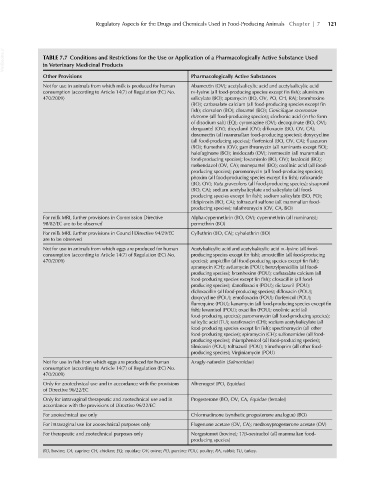
VetBooks.ir TABLE 7.7 Conditions and Restrictions for the Use or Application of a Pharmacologically Active Substance Used
in Veterinary Medicinal Products
Other Provisions Pharmacologically Active Substances
Not for use in animals from which milk is produced for human Abamectin (OV); acetylsalicylic acid and acetylsalicylic acid
consumption (according to Article 14(7) of Regulation (EC) No. DL-lysine (all food-producing species except fin fish); aluminum
470/2009) salicylate (BO); apramycin (BO, OV, PO, CH, RA); bromhexine
(BO); carbasalate calcium (all food-producing species except fin
fish); clorsulon (BO); closantel (BO); Cimicifugae racemosae
rhizome (all food-producing species); clodronic acid (in the form
of disodium salt) (EQ); cyromazine (OV); decoquinate (BO, OV);
derquantel (OV); dicyclanil (OV); difloxacin (BO, OV, CA);
doramectin (all mammalian food-producing species); doxycycline
(all food-producing species); florfenicol (BO, OV, CA); fluazuron
(BO); flumethrin (OV); gamithromycin (all ruminants except BO);
halofuginone (BO); imidocarb (OV); ivermectin (all mammalian
food-producing species); levamisole (BO, OV); lasalocid (BO);
mebendazol (OV, CA); monepantel (BO); oxolinic acid (all food-
producing species); paromomycin (all food-producing species);
phoxim (all food-producing species except fin fish); rafoxanide
(BO, OV); Ruta graveolens (all food-producing species); sisapronil
(BO, CA); sodium acetylsalicylate and salicylate (all food-
producing species except fin fish); sodium salicylate (BO, PO);
tildipirosin (BO, CA); toltrazuril sulfone (all mammalian food-
producing species); tulathromycin (OV, CA, BO)
For milk MRL further provisions in Commission Directive Alpha-cypermethrin (BO, OV); cypermethrin (all ruminants);
98/82/EC are to be observed permethrin (BO)
For milk MRL further provisions in Council Directive 94/29/EC Cyfluthrin (BO, CA); cyhalothrin (BO)
are to be observed
Not for use in animals from which eggs are produced for human Acetylsalicylic acid and acetylsalicylic acid DL-lysine (all food-
consumption (according to Article 14(7) of Regulation (EC) No. producing species except fin fish); amoxicillin (all food-producing
470/2009) species); ampicillin (all food-producing species except fin fish);
apramycin (CH); avilamycin (POU); benzylpenicillin (all food-
producing species); bromhexine (POU); carbasalate calcium (all
food-producing species except fin fish); cloxacillin (all food-
producing species); danofloxacin (POU); diclazuril (POU);
dicloxacillin (all food-producing species); difloxacin (POU);
doxycycline (POU); enrofloxacin (POU); florfenicol (POU);
flumequine (POU); kanamycin (all food-producing species except fin
fish); levamisol (POU); oxacillin (POU); oxolinic acid (all
food-producing species); paromomycin (all food-producing species);
salicylic acid (TU); sarafloxacin (CH); sodium acetylsalicylate (all
food-producing species except fin fish); spectinomycin (all other
food-producing species); spiramycin (CH); sulfonamides (all food-
producing species); thiamphenicol (all food-producing species);
tilmicosin (POU); toltrazuril (POU); trimethoprim (all other food-
producing species); Virginiamycin (POU)
Not for use in fish from which eggs are produced for human Azagly-nafarelin (Salmonidae)
consumption (according to Article 14(7) of Regulation (EC) No.
470/2009)
Only for zootechnical use and in accordance with the provisions Altrenogest (PO, Equidae)
of Directive 96/22/EC
Only for intravaginal therapeutic and zootechnical use and in Progesterone (BO, OV, CA, Equidae (female))
accordance with the provisions of Directive 96/22/EC
For zootechnical use only Chlormadinone (synthetic progesterone analogue) (BO)
For intravaginal use for zootechnical purposes only Flugestone acetate (OV, CA); medroxyprogesterone acetate (OV)
For therapeutic and zootechnical purposes only Norgestomet (bovine); 17β-oestradiol (all mammalian food-
producing species)
BO, bovine; CA, caprine; CH, chicken; EQ, equidae; OV, ovine; PO, porcine; POU, poultry; RA, rabbit; TU, turkey.