Page 606 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 606
Toxicity of Fungicides Chapter | 45 571
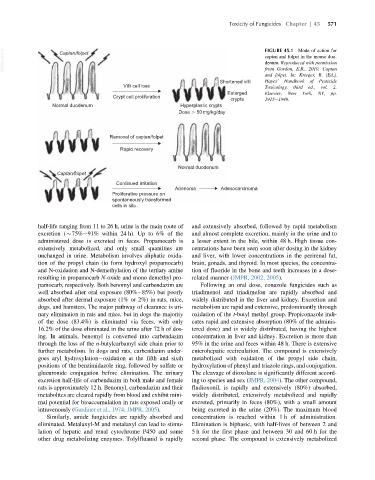
VetBooks.ir Captan/folpet FIGURE 45.1 Mode of action for
captan and folpet in the mouse duo-
denum. Reproduced with permission
from Gordon, E.B., 2010. Captan
and folpet. In: Krieger, R. (Ed.),
Shortened villi Hayes’ Handbook of Pesticide
Villi cell loss Toxicology, third ed., vol. 2.
Enlarged Elsevier, New York, NY, pp.
Crypt cell proliferation
crypts 1915 1949.
Normal duodenum Hyperplastic crypts
Dose 50 mg/kg/day
Removal of captan/folpet
Rapid recovery
Normal duodenum
Captan/folpet
Continued irritation
Adenoma Adenocarcinoma
Proliferative pressure on
spontaneously transformed
cells in situ.
half-life ranging from 11 to 26 h, urine is the main route of and extensively absorbed, followed by rapid metabolism
excretion (B75% 91% within 24 h). Up to 6% of the and almost complete excretion, mainly in the urine and to
administered dose is excreted in feces. Propamocarb is a lesser extent in the bile, within 48 h. High tissue con-
extensively metabolized, and only small quantities are centrations have been seen soon after dosing in the kidney
unchanged in urine. Metabolism involves aliphatic oxida- and liver, with lower concentrations in the perirenal fat,
tion of the propyl chain (to form hydroxyl propamocarb) brain, gonads, and thyroid. In most species, the concentra-
and N-oxidation and N-demethylation of the tertiary amine tion of fluoride in the bone and teeth increases in a dose-
resulting in propamocarb N-oxide and mono demethyl pro- related manner (JMPR, 2002, 2005).
pamocarb, respectively. Both benomyl and carbendazim are Following an oral dose, conazole fungicides such as
well absorbed after oral exposure (80% 85%) but poorly triadimenol and triadimefon are rapidly absorbed and
absorbed after dermal exposure (1% or 2%) in rats, mice, widely distributed in the liver and kidney. Excretion and
dogs, and hamsters. The major pathway of clearance is uri- metabolism are rapid and extensive, predominantly through
nary elimination in rats and mice, but in dogs the majority oxidation of the t-butyl methyl group. Propiconazole indi-
of the dose (83.4%) is eliminated via feces, with only cates rapid and extensive absorption (80% of the adminis-
16.2% of the dose eliminated in the urine after 72 h of dos- tered dose) and is widely distributed, having the highest
ing. In animals, benomyl is converted into carbendazim concentration in liver and kidney. Excretion is more than
through the loss of the n-butylcarbamyl side chain prior to 95% in the urine and feces within 48 h. There is extensive
further metabolism. In dogs and rats, carbendazim under- enterohepatic recirculation. The compound is extensively
goes aryl hydroxylation oxidation at the fifth and sixth metabolized with oxidation of the propyl side chain,
positions of the benzimidazole ring, followed by sulfate or hydroxylation of phenyl and triazole rings, and conjugation.
glucuronide conjugation before elimination. The urinary The cleavage of dioxolane is significantly different accord-
excretion half-life of carbendazim in both male and female ing to species and sex (JMPR, 2004). The other compound,
rats is approximately 12 h. Benomyl, carbendazim and their fludioxonil, is rapidly and extensively (80%) absorbed,
metabolites are cleared rapidly from blood and exhibit mini- widely distributed, extensively metabolized and rapidly
mal potential for bioaccumulation in rats exposed orally or excreted, primarily in feces (80%), with a small amount
intravenously (Gardiner et al., 1974; JMPR, 2005). being excreted in the urine (20%). The maximum blood
Similarly, amide fungicides are rapidly absorbed and concentration is reached within 1 h of administration.
eliminated. Metalaxyl-M and metalaxyl can lead to stimu- Elimination is biphasic, with half-lives of between 2 and
lation of hepatic and renal cytochrome P450 and some 5 h for the first phase and between 30 and 60 h for the
other drug metabolizing enzymes. Tolylfluanid is rapidly second phase. The compound is extensively metabolized