Page 621 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 621
586 SECTION | VIII Rodenticides
VetBooks.ir to be a little more effective than warfarin against R. nor- than 10 mg/L in water. It is more toxic than warfarin, but
In spite of its low toxicity, coumatetralyl is reported
less palatable. Difenacoum is still effective against many
populations of warfarin-resistant rats (Desideri et al.,
vegicus, apparently due to a higher palatability.
Coumatetralyl was introduced after the detection of 1979), but resistance may be developing in the United
warfarin-resistant rat populations, and showed consider- Kingdom (Greaves et al., 1982).
able success for a number of years, but resistant pests
have been reported in the United Kingdom and Denmark Warfarin
(Rowe and Redfern,1968).
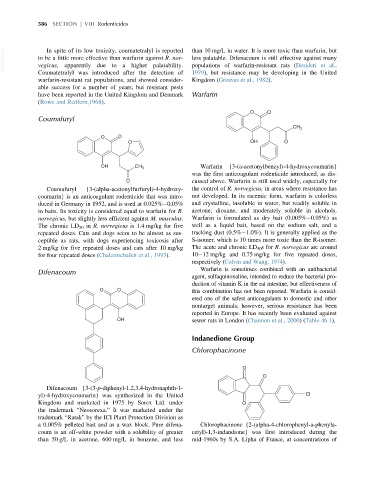
O O
Coumafuryl
CH 3
O O
O OH O
OH CH 3 Warfarin {3-(a-acetonylbenzyl)-4-hydroxycoumarin}
was the first anticoagulant rodenticide introduced, as dis-
O cussed above. Warfarin is still used widely, especially for
Coumafuryl {3-(alpha-acetonylfurfuryl)-4-hydroxy- the control of R. norvegicus, in areas where resistance has
coumarin} is an anticoagulant rodenticide that was intro- not developed. In its racemic form, warfarin is colorless
duced in Germany in 1952, and is used at 0.025% 0.05% and crystalline, insoluble in water, but readily soluble in
in baits. Its toxicity is considered equal to warfarin for R. acetone, dioxane, and moderately soluble in alcohols.
norvegicus, but slightly less efficient against M. musculus. Warfarin is formulated as dry bait (0.005% 0.05%) as
The chronic LD 50 in R. norvegicus is 1.4 mg/kg for five well as a liquid bait, based on the sodium salt, and a
repeated doses. Cats and dogs seem to be almost as sus- tracking dust (0.5% 1.0%). It is generally applied as the
ceptible as rats, with dogs experiencing toxicosis after S-isomer, which is 10 times more toxic than the R-isomer.
2 mg/kg for five repeated doses and cats after 10 mg/kg The acute and chronic LD 50 sfor R. norvegicus are around
for four repeated doses (Chalermchaikit et al., 1993). 10 12 mg/kg and 0.75 mg/kg for five repeated doses,
respectively (Colvin and Wang, 1974).
Warfarin is sometimes combined with an antibacterial
Difenacoum
agent, sulfaquinoxaline, intended to reduce the bacterial pro-
duction of vitamin K in the rat intestine, but effectiveness of
O O this combination has not been reported. Warfarin is consid-
ered one of the safest anticoagulants to domestic and other
nontarget animals; however, serious resistance has been
reported in Europe. It has recently been evaluated against
OH sewer rats in London (Channon et al., 2000)(Table 46.1).
Indanedione Group
Chlorophacinone
O
O
Difenacoum {3-(3-p-diphenyl-1,2,3,4-hydronaphth-1-
yl)-4-hydroxycoumarin} was synthesized in the United Cl
Kingdom and marketed in 1975 by Sorex Ltd. under O
the trademark “Neosorexa.” It was marketed under the
trademark “Ratak” by the ICI Plant Protection Division as
a 0.005% pelleted bait and as a wax block. Pure difena- Chlorophacinone {2-(alpha-4-chlorophenyl-a-phenyla-
coum is an off-white powder with a solubility of greater cetyl)-1,3-indandione} was first introduced during the
than 50 g/L in acetone, 600 mg/L in benzene, and less mid-1960s by S.A. Lipha of France, at concentrations of