Page 622 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 622
Anticoagulant Chapter | 46 587
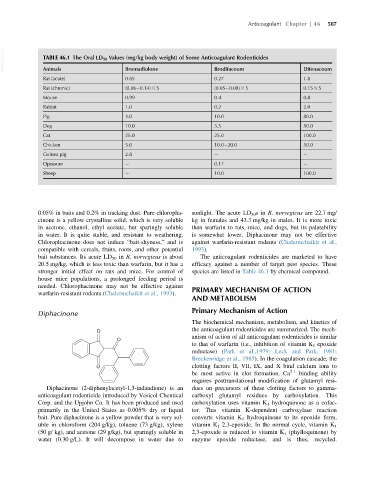
VetBooks.ir TABLE 46.1 The Oral LD 50 Values (mg/kg body weight) of Some Anticoagulant Rodenticides Difenacoum
Animals
Bromadiolone
Brodifacoum
Rat (acute) 0.65 0.27 1.8
Rat (chronic) (0.06 0.14) 3 5 (0.05 0.08) 3 5 0.15 3 5
Mouse 0.99 0.4 0.8
Rabbit 1.0 0.2 2.0
Pig 3.0 10.0 80.0
Dog 10.0 3.5 50.0
Cat 25.0 25.0 100.0
Chicken 5.0 10.0 20.0 50.0
Guinea pig 2.8
Opossum 0.17
Sheep 10.0 100.0
0.05% in baits and 0.2% in tracking dust. Pure chloropha- sunlight. The acute LD 50 sin R. norvegicus are 22.7 mg/
cinone is a yellow crystalline solid, which is very soluble kg in females and 43.3 mg/kg in males. It is more toxic
in acetone, ethanol, ethyl acetate, but sparingly soluble than warfarin to rats, mice, and dogs, but its palatability
in water. It is quite stable, and resistant to weathering. is somewhat lower. Diphacinone may not be effective
Chlorophacinone does not induce “bait-shyness,” and is against warfarin-resistant rodents (Chalermchaikit et al.,
compatible with cereals, fruits, roots, and other potential 1993).
bait substances. Its acute LD 50 in R. norvegicus is about The anticoagulant rodenticides are marketed to have
20.5 mg/kg, which is less toxic than warfarin, but it has a efficacy against a number of target pest species. These
stronger initial effect on rats and mice. For control of species are listed in Table 46.1 by chemical compound.
house mice populations, a prolonged feeding period is
needed. Chlorophacinone may not be effective against PRIMARY MECHANISM OF ACTION
warfarin-resistant rodents (Chalermchaikit et al., 1993).
AND METABOLISM
Primary Mechanism of Action
Diphacinone
The biochemical mechanism, metabolism, and kinetics of
O the anticoagulant rodenticides are summarized. The mech-
anism of action of all anticoagulant rodenticides is similar
O
to that of warfarin (i.e., inhibition of vitamin K 1 epoxide
reductase) (Park et al.,1979; Leck and Park, 1981;
Breckenridge et al., 1985). In the coagulation cascade, the
O clotting factors II, VII, IX, and X bind calcium ions to
be most active in clot formation. Ca 21 binding ability
requires posttranslational modification of glutamyl resi-
Diphacinone (2-diphenylacetyl-1,3-indandione) is an dues on precursors of these clotting factors to gamma-
anticoagulant rodenticide introduced by Vesicol Chemical carboxyl glutamyl residues by carboxylation. This
Corp. and the Upjohn Co. It has been produced and used carboxylation uses vitamin K 1 hydroquinone as a cofac-
primarily in the United States as 0.005% dry or liquid tor. This vitamin K-dependent carboxylase reaction
bait. Pure diphacinone is a yellow powder that is very sol- converts vitamin K 1 hydroquinone to its epoxide form,
uble in chloroform (204 g/kg), toluene (73 g/kg), xylene vitamin K 1 2,3-epoxide. In the normal cycle, vitamin K 1
(50 g/ kg), and acetone (29 g/kg), but sparingly soluble in 2,3-epoxide is reduced to vitamin K 1 (phylloquinone) by
water (0.30 g/L). It will decompose in water due to enzyme epoxide reductase, and is thus, recycled.