Page 1370 - Small Animal Internal Medicine, 6th Edition
P. 1370
1342 PART XIII Hematology
TABLE 82.1 provides relevant clinicopathologic information in most
patients with anemia.
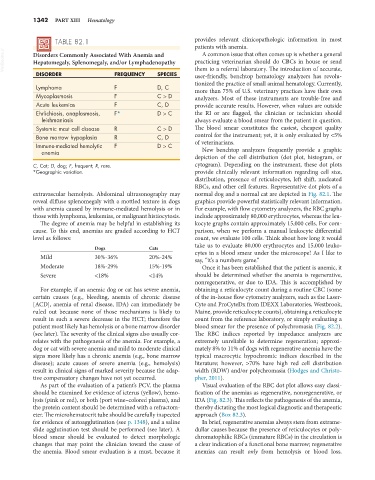
VetBooks.ir Disorders Commonly Associated With Anemia and practicing veterinarian should do CBCs in house or send
A common issue that often comes up is whether a general
Hepatomegaly, Splenomegaly, and/or Lymphadenopathy
DISORDER FREQUENCY SPECIES them to a referral laboratory. The introduction of accurate,
user-friendly, benchtop hematology analyzers has revolu-
tionized the practice of small animal hematology. Currently,
Lymphoma F D, C more than 75% of U.S. veterinary practices have their own
Mycoplasmosis F C > D analyzers. Most of these instruments are trouble-free and
Acute leukemias F C, D provide accurate results. However, when values are outside
Ehrlichiosis, anaplasmosis, F* D > C the RI or are flagged, the clinician or technician should
leishmaniasis always evaluate a blood smear from the patient in question.
Systemic mast cell disease R C > D The blood smear constitutes the easiest, cheapest quality
Bone marrow hypoplasia R C, D control for the instrument; yet, it is only evaluated by <5%
Immune-mediated hemolytic F D > C of veterinarians.
anemia New benchtop analyzers frequently provide a graphic
depiction of the cell distribution (dot plot, histogram, or
C, Cat; D, dog; F, frequent; R, rare. cytogram). Depending on the instrument, these dot plots
*Geographic variation. provide clinically relevant information regarding cell size,
distribution, presence of reticulocytes, left shift, nucleated
RBCs, and other cell features. Representative dot plots of a
extravascular hemolysis. Abdominal ultrasonography may normal dog and a normal cat are depicted in Fig. 82.1. The
reveal diffuse splenomegaly with a mottled texture in dogs graphics provide powerful statistically relevant information.
with anemia caused by immune-mediated hemolysis or in For example, with flow cytometry analyzers, the RBC graphs
those with lymphoma, leukemias, or malignant histiocytosis. include approximately 80,000 erythrocytes, whereas the leu-
The degree of anemia may be helpful in establishing its kocyte graphs contain approximately 15,000 cells. For com-
cause. To this end, anemias are graded according to HCT parison, when we perform a manual leukocyte differential
level as follows: count, we evaluate 100 cells. Think about how long it would
Dogs Cats take us to evaluate 80,000 erythrocytes and 15,000 leuko-
Mild 30%-36% 20%-24% cytes in a blood smear under the microscope! As I like to
say, “it’s a numbers game.”
Moderate 18%-29% 15%-19% Once it has been established that the patient is anemic, it
Severe <18% <14% should be determined whether the anemia is regenerative,
nonregenerative, or due to IDA. This is accomplished by
For example, if an anemic dog or cat has severe anemia, obtaining a reticulocyte count during a routine CBC (some
certain causes (e.g., bleeding, anemia of chronic disease of the in-house flow cytometry analyzers, such as the Laser-
[ACD], anemia of renal disease, IDA) can immediately be Cyte and ProCyteDx from IDEXX Laboratories, Westbrook,
ruled out because none of those mechanisms is likely to Maine, provide reticulocyte counts), obtaining a reticulocyte
result in such a severe decrease in the HCT; therefore the count from the reference laboratory, or simply evaluating a
patient most likely has hemolysis or a bone marrow disorder blood smear for the presence of polychromasia (Fig. 82.2).
(see later). The severity of the clinical signs also usually cor- The RBC indices reported by impedance analyzers are
relates with the pathogenesis of the anemia. For example, a extremely unreliable to determine regeneration; approxi-
dog or cat with severe anemia and mild to moderate clinical mately 8% to 11% of dogs with regenerative anemia have the
signs more likely has a chronic anemia (e.g., bone marrow typical macrocytic hypochromic indices described in the
disease); acute causes of severe anemia (e.g., hemolysis) literature; however, >70% have high red cell distribution
result in clinical signs of marked severity because the adap- width (RDW) and/or polychromasia (Hodges and Christo-
tive compensatory changes have not yet occurred. pher, 2011).
As part of the evaluation of a patient’s PCV, the plasma Visual evaluation of the RBC dot plot allows easy classi-
should be examined for evidence of icterus (yellow), hemo- fication of the anemias as regenerative, nonregenerative, or
lysis (pink or red), or both (port wine–colored plasma), and IDA (Fig. 82.3). This reflects the pathogenesis of the anemia,
the protein content should be determined with a refractom- thereby dictating the most logical diagnostic and therapeutic
eter. The microhematocrit tube should be carefully inspected approach (Box 82.3).
for evidence of autoagglutination (see p. 1348), and a saline In brief, regenerative anemias always stem from extrame-
slide agglutination test should be performed (see later). A dullar causes because the presence of reticulocytes or poly-
blood smear should be evaluated to detect morphologic chromatophilic RBCs (immature RBCs) in the circulation is
changes that may point the clinician toward the cause of a clear indication of a functional bone marrow; regenerative
the anemia. Blood smear evaluation is a must, because it anemias can result only from hemolysis or blood loss.