Page 397 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 397
CHAPTER 20 Melanoma 375
P P
VetBooks.ir
L R L R
A B
A A
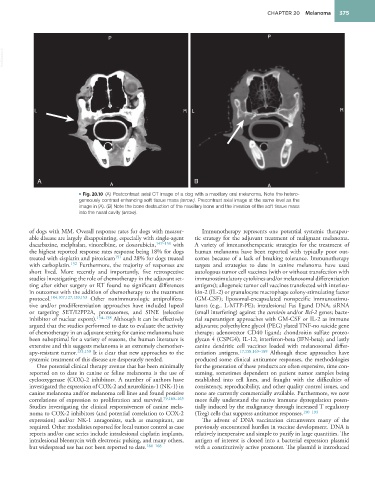
• Fig. 20.10 (A) Postcontrast axial CT image of a dog with a maxillary oral melanoma. Note the hetero-
geneously contrast enhancing soft tissue mass (arrow). Precontrast axial image at the same level as the
image in (A). (B) Note the bone destruction of the maxillary bone and the invasion of the soft tissue mass
into the nasal cavity (arrow).
of dogs with MM. Overall response rates for dogs with measur- Immunotherapy represents one potential systemic therapeu-
able disease are largely disappointing, especially with single-agent tic strategy for the adjuvant treatment of malignant melanoma.
dacarbazine, melphalan, vinorelbine, or doxorubicin, 147–150 with A variety of immunotherapeutic strategies for the treatment of
the highest reported response rates response being 18% for dogs human melanoma have been reported with typically poor out-
treated with cisplatin and piroxicam 151 and 28% for dogs treated comes because of a lack of breaking tolerance. Immunotherapy
with carboplatin. 152 Furthermore, the majority of responses are targets and strategies to date in canine melanoma have used
short lived. More recently and importantly, five retrospective autologous tumor cell vaccines (with or without transfection with
studies investigating the role of chemotherapy in the adjuvant set- immunostimulatory cytokines and/or melanosomal differentiation
ting after either surgery or RT found no significant differences antigens); allogeneic tumor cell vaccines transfected with interleu-
in outcomes with the addition of chemotherapy to the treatment kin-2 (IL-2) or granulocyte macrophage colony-stimulating factor
protocol. 104,107,127,139,153 Other nonimmunologic antiprolifera- (GM-CSF); liposomal-encapsulated nonspecific immunostimu-
tive and/or prodifferentiation approaches have included lupeol lators (e.g.. L-MTP-PE); intralesional Fas ligand DNA; siRNA
or targeting SET/I2PP2A, proteasomes, and SINE (selective (small interfering) against the survivin and/or Bcl-2 genes; bacte-
inhibitor of nuclear export). 154–158 Although it can be effectively rial superantigen approaches with GM-CSF or IL-2 as immune
argued that the studies performed to date to evaluate the activity adjuvants; polyethylene glycol (PEG) ylated TNF-no suicide gene
of chemotherapy in an adjuvant setting for canine melanoma have therapy; adenovector CD40 ligand; chondroitin sulfate proteo-
been suboptimal for a variety of reasons, the human literature is glycan 4 (CSPG4); IL-12; interferon-beta (IFN-beta); and lastly
extensive and this suggests melanoma is an extremely chemother- canine dendritic cell vaccines loaded with melanosomal differ-
apy-resistant tumor. 135,159 It is clear that new approaches to the entiation antigens. 17,158,169–189 Although these approaches have
systemic treatment of this disease are desperately needed. produced some clinical antitumor responses, the methodologies
One potential clinical therapy avenue that has been minimally for the generation of these products are often expensive, time con-
reported on to date in canine or feline melanoma is the use of suming, sometimes dependent on patient tumor samples being
cyclooxygenase (COX)-2 inhibitors. A number of authors have established into cell lines, and fraught with the difficulties of
investigated the expression of COX-2 and neurokinin-1 (NK-1) in consistency, reproducibility, and other quality control issues, and
canine melanoma and/or melanoma cell lines and found positive none are currently commercially available. Furthermore, we now
correlations of expression to proliferation and survival. 79,160–165 more fully understand the native immune dysregulation poten-
Studies investigating the clinical responsiveness of canine mela- tially induced by the malignancy through increased T regulatory
noma to COX-2 inhibitors (and potential correlation to COX-2 (Treg) cells that suppress antitumor responses. 190–193
expression) and/or NK-1 antagonists, such as maropitant, are The advent of DNA vaccination circumvents many of the
required. Other modalities reported for local tumor control as case previously encountered hurdles in vaccine development. DNA is
reports and/or case series include intralesional cisplatin implants, relatively inexpensive and simple to purify in large quantities. The
intralesional bleomycin with electronic pulsing, and many others, antigen of interest is cloned into a bacterial expression plasmid
but widespread use has not been reported to date. 166–168 with a constitutively active promoter. The plasmid is introduced