Page 565 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 565
CHAPTER 25 Tumors of the Skeletal System 543
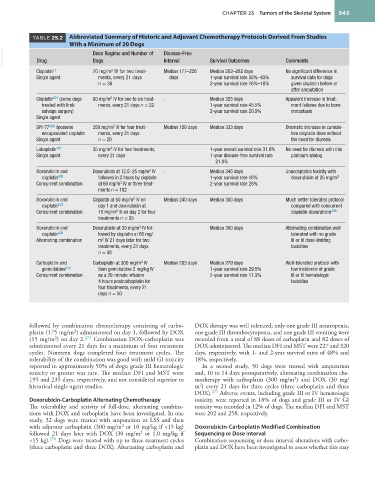
TABLE 25.2 Abbreviated Summary of Historic and Adjuvant Chemotherapy Protocols Derived From Studies
With a Minimum of 20 Dogs
VetBooks.ir Drug Dose Regime and Number of Disease-Free Survival Outcomes Comments
Dogs
Interval
2
Cisplatin 17 70 mg/m IV for two treat- Median 177–226 Median 262–282 days No significant difference in
Single agent ments, every 21 days days 1-year survival rate 38%–43% survival data for dogs
n = 36 2-year survival rate 16%–18% given cisplatin before or
after amputation
2
Cisplatin 201 (some dogs 60 mg/m IV for one to six treat- - Median 325 days Apparent increase in treat-
treated with limb ments, every 21 days n = 22 1-year survival rate 45.5% ment failures due to bone
salvage surgery) 2-year survival rate 20.9% metastasis
Single agent
SPI-77 269 liposome 350 mg/m IV for four treat- Median 156 days Median 333 days Dramatic increase in cumula-
2
encapsulated cisplatin ments, every 21 days tive cisplatin dose without
Single agent n = 20 the need for diuresis
Lobaplatin 407 35 mg/m IV for four treatments, 1-year overall survival rate 31.8% No need for diuresis with this
2
Single agent every 21 days 1-year disease-free survival rate platinum analog
21.8%
Doxorubicin and Doxorubicin at 12.5–25 mg/m IV - Median 345 days Unacceptable toxicity with
2
cisplatin 408 followed in 2 hours by cisplatin 1-year survival rate 48% doxorubicin at 25 mg/m 2
2
Concurrent combination at 60 mg/m IV or three treat- 2-year survival rate 28%
ments n = 102
2
Doxorubicin and Cisplatin at 50 mg/m IV on Median 240 days Median 300 days Much better tolerated protocol
cisplatin 273 day 1 and doxorubicin at compared with concurrent
2
Concurrent combination 15 mg/m IV on day 2 for four cisplatin-doxorubicin 408
treatments n = 35
2
Doxorubicin and Doxorubicin at 30 mg/m IV fol- Median 300 days Alternating combination well
cisplatin 409 lowed by cisplatin at 60 mg/ tolerated with no grade
2
Alternating combination m IV 21 days later for two III or IV dose-limiting
treatments, every 21 days toxicities
n = 38
2
Carboplatin and Carboplatin at 300 mg/m IV Median 203 days Median 279 days Well-tolerated protocol with
gemcitabine 410 then gemcitabine 2 mg/kg IV 1-year survival rate 29.5% low incidence of grade
Concurrent combination as a 20-minute infusion 2-year survival rate 11.3% III or IV hematologic
4 hours postcarboplatin for toxicities
four treatments, every 21
days n = 50
followed by combination chemotherapy consisting of carbo- DOX therapy was well tolerated; only one grade III neutropenia,
2
platin (175 mg/m ) administered on day 1, followed by DOX one grade III thrombocytopenia, and one grade III vomiting were
(15 mg/m ) on day 2. 275 Combination DOX-carboplatin was recorded from a total of 88 doses of carboplatin and 82 doses of
2
administered every 21 days for a maximum of four treatment DOX administered. The median DFI and MST were 227 and 320
cycles. Nineteen dogs completed four treatment cycles. The days, respectively, with 1- and 2-year survival rates of 48% and
tolerability of the combination was good with mild GI toxicity 18%, respectively.
reported in approximately 50% of dogs; grade III hematologic In a second study, 50 dogs were treated with amputation
toxicity or greater was rare. The median DFI and MST were and, 10 to 14 days postoperatively, alternating combination che-
2
195 and 235 days, respectively, and not considered superior to motherapy with carboplatin (300 mg/m ) and DOX (30 mg/
2
historical single-agent studies. m ) every 21 days for three cycles (three carboplatin and three
DOX). 277 Adverse events, including grade III or IV hematologic
Doxorubicin-Carboplatin Alternating Chemotherapy toxicity, were reported in 18% of dogs and grade III or IV GI
The tolerability and activity of full-dose, alternating combina- toxicity was recorded in 12% of dogs. The median DFI and MST
tions with DOX and carboplatin have been investigated. In one were 202 and 258, respectively.
study, 32 dogs were treated with amputation or LSS and then
with adjuvant carboplatin (300 mg/m or 10 mg/kg if <15 kg) Doxorubicin-Carboplatin Modified Combination
2
followed 21 days later with DOX (30 mg/m or 1.0 mg/kg if Sequencing or Dose Interval
2
<15 kg). 276 Dogs were treated with up to three treatment cycles Combination sequencing or dose interval alterations with carbo-
(three carboplatin and three DOX). Alternating carboplatin and platin and DOX have been investigated to assess whether this may