Page 109 - Veterinary Immunology, 10th Edition
P. 109
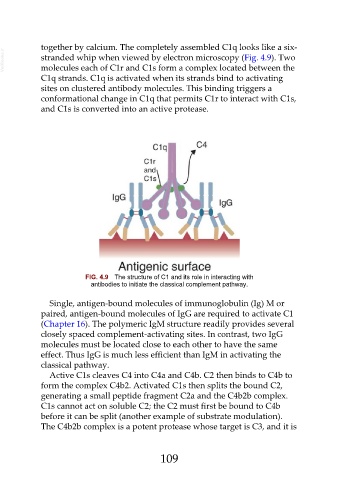
together by calcium. The completely assembled C1q looks like a six-
VetBooks.ir stranded whip when viewed by electron microscopy (Fig. 4.9). Two
molecules each of C1r and C1s form a complex located between the
C1q strands. C1q is activated when its strands bind to activating
sites on clustered antibody molecules. This binding triggers a
conformational change in C1q that permits C1r to interact with C1s,
and C1s is converted into an active protease.
FIG. 4.9 The structure of C1 and its role in interacting with
antibodies to initiate the classical complement pathway.
Single, antigen-bound molecules of immunoglobulin (Ig) M or
paired, antigen-bound molecules of IgG are required to activate C1
(Chapter 16). The polymeric IgM structure readily provides several
closely spaced complement-activating sites. In contrast, two IgG
molecules must be located close to each other to have the same
effect. Thus IgG is much less efficient than IgM in activating the
classical pathway.
Active C1s cleaves C4 into C4a and C4b. C2 then binds to C4b to
form the complex C4b2. Activated C1s then splits the bound C2,
generating a small peptide fragment C2a and the C4b2b complex.
C1s cannot act on soluble C2; the C2 must first be bound to C4b
before it can be split (another example of substrate modulation).
The C4b2b complex is a potent protease whose target is C3, and it is
109