Page 485 - Veterinary Immunology, 10th Edition
P. 485
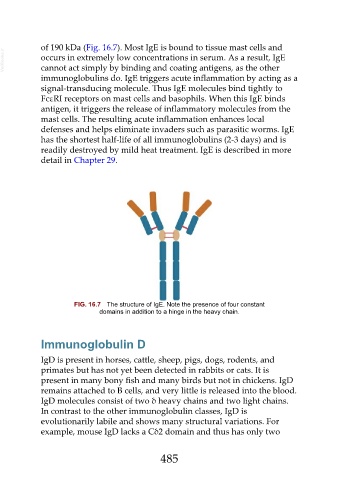
of 190 kDa (Fig. 16.7). Most IgE is bound to tissue mast cells and
VetBooks.ir occurs in extremely low concentrations in serum. As a result, IgE
cannot act simply by binding and coating antigens, as the other
immunoglobulins do. IgE triggers acute inflammation by acting as a
signal-transducing molecule. Thus IgE molecules bind tightly to
FcεRI receptors on mast cells and basophils. When this IgE binds
antigen, it triggers the release of inflammatory molecules from the
mast cells. The resulting acute inflammation enhances local
defenses and helps eliminate invaders such as parasitic worms. IgE
has the shortest half-life of all immunoglobulins (2-3 days) and is
readily destroyed by mild heat treatment. IgE is described in more
detail in Chapter 29.
FIG. 16.7 The structure of IgE. Note the presence of four constant
domains in addition to a hinge in the heavy chain.
Immunoglobulin D
IgD is present in horses, cattle, sheep, pigs, dogs, rodents, and
primates but has not yet been detected in rabbits or cats. It is
present in many bony fish and many birds but not in chickens. IgD
remains attached to B cells, and very little is released into the blood.
IgD molecules consist of two δ heavy chains and two light chains.
In contrast to the other immunoglobulin classes, IgD is
evolutionarily labile and shows many structural variations. For
example, mouse IgD lacks a Cδ2 domain and thus has only two
485