Page 218 - Medicinal Chemistry Self Assessment
P. 218
Please replace the indicated structure in Question 6 in both 1.21 and 2.21 (the one that is part of the question)
with the one below. Both structures had an identical error.
Metabolite of
phenobarbital
secobarbital
butabarbital
Chapters 1.21 and 2.21 Metabolite of Metabolite of
Chapter 2.22
Please replace the structure for the answer to Question 3 in Chapter 2.22 with the one below.
2.22 Pravastatin and Fluvastatin 207
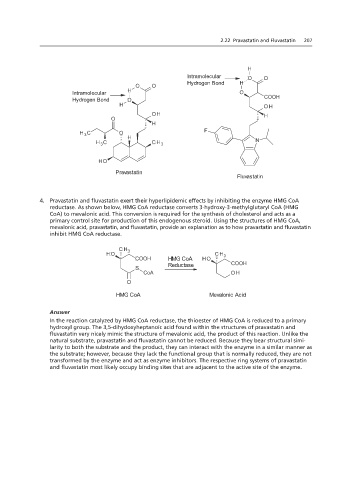
3. The normal pK a range for at the high end of the normal range.
Answer
Intramolecular
The presence or absence of its pK a value. Hydrogen Bond
Intramolecular
Hydrogen Bond H
Intramolecular O O
Hydrogen Bone H
O O
Intramolecular H O
Hydrogen Bone O COOH
H OH
OH H
O
H
H 3 C O H F
CH
H 3 C Pravastatin 3 N
Fluvastatin
H O
4. Pravastatin and fluvastatin exert their hyperlipidemic effects by inhibiting the enzyme HMG CoA
Pravastatin
reductase. As shown below, HMG CoA reductase converts 3-hydroxy-3-methylglutaryl CoA (HMG
Fluvastatin
CoA) to mevalonic acid. This conversion is required for the synthesis of cholesterol and acts as a
primary control site for production of this endogenous steroid. Using the structures of HMG CoA,
mevalonic acid, pravastatin, and fluvastatin, provide an explanation as to how pravastatin and fluvastatin
4. Pravastatin and fluvastatin fluvastatin, provide an d fluvastain inhibit HMG CoA reductase.
inhibit HMG CoA reductase.
HMG CoA
Reductase
HMG CoA Mevalonic Acid
Answer
In the reaction catalyzed by HMG CoA reductase, the thioester of HMG CoA is reduced to a primary
hydroxyl group. The 3,5-dihydoxyheptanoic acid found within the structures of pravastatin and
fluvastatin very nicely mimic the structure of mevalonic acid, the product of this reaction. Unlike the
natural substrate, pravastatin and fluvastatin cannot be reduced. Because they bear structural simi-
larity to both the substrate and the product, they can interact with the enzyme in a similar manner as
the substrate; however, because they lack the functional group that is normally reduced, they are not
transformed by the enzyme and act as enzyme inhibitors. The respective ring systems of pravastatin
and fluvastatin most likely occupy binding sites that are adjacent to the active site of the enzyme.