Page 223 - Medicinal Chemistry Self Assessment
P. 223
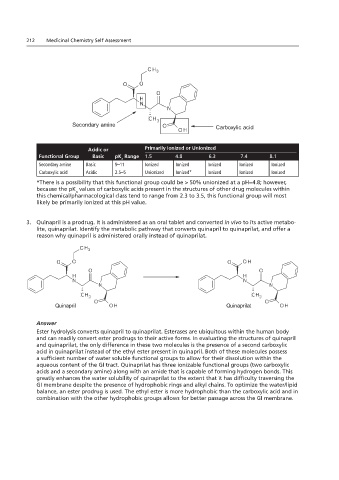
2.23 Quinapril
Shown below is the structure of quinapril. It is an groups are identified.
B
H
H
N
2.23 Quinapril A O 3 C O O D N
C
CH
Shown below is the structure of quinapril. It is an groups are identified.
3
O OH
B E
H 3 C
O
212 1. Using the table below, identify the five explanation for your response.
Medicinal Chemistry Self Assessment O
D
2. Using the h functional ionized or unionized at pH environments of 1.5, 4.8, 6.3, 7.4, and 8.1.
A
O
H
N
N
C
CH 3
O
OH
E
1. Using the table below, identify the five explanation for your response.
2. Using the h functional ionized or unionized at pH environments of 1.5, 4.8, 6.3, 7.4, and 8.1.
Secondary amine
Carboxylic acid
Acidic or Primarily Ionized or Unionized
Functional Group Basic pK Range 1.5 4.8 6.3 7.4 8.1
a
3. Quinapril is a is administered orally instead of quinaprilat.
Secondary amine Basic 9–11 Ionized Ionized Ionized Ionized Ionized
Carboxylic acid Acidic 2.5–5 Unionized Ionized* Ionized Ionized Ionized
*There is a possibility that this functional group could be > 50% unionized at a pH=4.8; however,
because the pK values of carboxylic acids present in the structures of other drug molecules within
a
Secondary amine
this chemical/pharmacological class tend to range from 2.3 to 3.5, this functional group will most
Carboxylic acid
likely be primarily ionized at this pH value.
3. Quinapril is a prodrug. It is administered as an oral tablet and converted in vivo to its active metabo-
3. Quinapril is a is administered orally instead of quinaprilat.
lite, quinaprilat. Identify the metabolic pathway that converts quinapril to quinaprilat, and offer a
reason why quinapril is administered orally instead of quinaprilat.
Quinapril Quinaprilat
Quinapril Quinaprilat
Answer
Ester hydrolysis converts quinapril to quinaprilat. Esterases are ubiquitous within the human body
and can readily convert ester prodrugs to their active forms. In evaluating the structures of quinapril
and quinaprilat, the only difference in these two molecules is the presence of a second carboxylic
acid in quinaprilat instead of the ethyl ester present in quinapril. Both of these molecules possess
a sufficient number of water soluble functional groups to allow for their dissolution within the
aqueous content of the GI tract. Quinaprilat has three ionizable functional groups (two carboxylic
acids and a secondary amine) along with an amide that is capable of forming hydrogen bonds. This
greatly enhances the water solubility of quinaprilat to the extent that it has difficulty traversing the
GI membrane despite the presence of hydrophobic rings and alkyl chains. To optimize the water/lipid
balance, an ester prodrug is used. The ethyl ester is more hydrophobic than the carboxylic acid and in
combination with the other hydrophobic groups allows for better passage across the GI membrane.