Page 246 - Medicinal Chemistry Self Assessment
P. 246
4. Zanamivir exerts its his, provide an explanation how zanamivir inhibits neuraminidase.
Glycosidic bond
4. Zanamivir exerts its his, provide an explanation how zanamivir inhibits neuraminidase.
N-Acetylsialic acid bound to glycoprotein Zanamivir
Answer:
2.27 Zanamivir and Oseltamivir 235
Zanamivir is a stable a cleavable glycosidic bond.
Structurally identical
to N-acetylsialic acid
(with exception of
the double bond)
Glycosidic bond
N-Acetylsialic acid bound to glycoprotein Zanamivir
Answer:
Lacks glycosidic bond
Zanamivir is a stable a cleavable glycosidic bond.
Zanamivir
Structurally identical
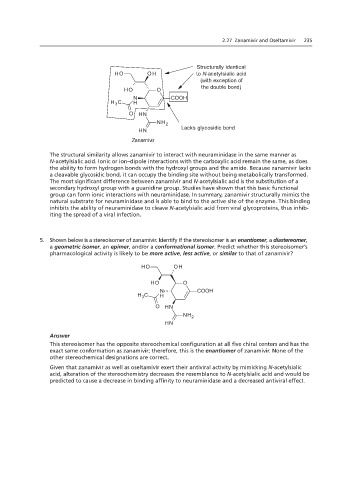
The structural similarity allows zanamivir to interact with neuraminidase in the same manner as
5. Shown below is a activity is likely to be more active, less active, or similar to that of zanamivir?
to N-acetylsialic acid
N-acetylsialic acid. Ionic or ion–dipole interactions with the carboxylic acid remain the same, as does
(with exception of
the ability to form hydrogen bonds with the hydroxyl groups and the amide. Because zanamivir lacks
the double bond)
a cleavable glycosidic bond, it can occupy the binding site without being metabolically transformed.
The most significant difference between zanamivir and N-acetylsialic acid is the substitution of a
secondary hydroxyl group with a guanidine group. Studies have shown that this basic functional
group can form ionic interactions with neuraminidase. In summary, zanamivir structurally mimics the
natural substrate for neuraminidase and is able to bind to the active site of the enzyme. This binding
inhibits the ability of neuraminidase to cleave N-acetylsialic acid from viral glycoproteins, thus inhib-
iting the spread of a viral infection. Lacks glycosidic bond
Zanamivir
5. Shown below is a stereoisomer of zanamivir. Identify if the stereoisomer is an enantiomer, a diastereomer,
5. Shown below is a activity is likely to be more active, less active, or similar to that of zanamivir?
a geometric isomer, an epimer, and/or a conformational isomer. Predict whether this stereoisomer’s
pharmacological activity is likely to be more active, less active, or similar to that of zanamivir?
6. Shown below is the structure of for oseltamivir
Answer
This stereoisomer has the opposite stereochemical configuration at all five chiral centers and has the
6. Shown below is the structure of for oseltamivir
exact same conformation as zanamivir; therefore, this is the enantiomer of zanamivir. None of the
other stereochemical designations are correct.
Given that zanamivir as well as oseltamivir exert their antiviral activity by mimicking N-acetylsialic
acid, alteration of the stereochemistry decreases the resemblance to N-acetylsialic acid and would be
predicted to cause a decrease in binding affinity to neuraminidase and a decreased antiviral effect.