Page 241 - Medicinal Chemistry Self Assessment
P. 241
2.26 Sorafenib
Because protein tyrosine kinases owth is instrumental in the generation of new blood vessels.
C
E F
A
B D
230 Medicinal Chemistry Self Assessment
Sorafenib
1. Conduct a to the questions that follow.
b. Determine which of the boxed functional groups (A–E) can interact with the side chains of the
amino acids found in each of the five binding pockets. Indicate the type of interaction(s) possible
2. Sorafenib interacts with in the local environment of the enzyme.
in the appropriate box. None is an acceptable answer. Assume that the drug and the amino acid
side chains are both at pH=7.4.
3. Nilotinib, another and Leu 298 /Val 299 /Phe 359 in each of the respective five binding pockets.
Leu /Val 289 A Asp /Glu 286 Thr 315 Met 318 Leu /Val /Phe 359
285
299
298
391
A Hydrophobic Ion–dipole (functional H-bonding (A) Hydrophobic Hydrophobic
group as the dipole) Dipole–dipole π-π Stacking
B C D E
B Hydrophobic None H-bonding (A) Hydrophobic Hydrophobic
van der Waals Dipole–dipole van der Waals
π-π Stacking
C None Ion–dipole (functional H-bonding (A+D) None None
group as the dipole) Dipole–dipole
D* None Ion–dipole (functional H-bonding (A+D) None None
group as the dipole) Dipole–dipole
E** Hydrophobic Ion–dipole (functional Nilotinib None Hydrophobic
H-bonding (A)
group as the dipole) Dipole–dipole π-π Stacking
* Aniline-like nitrogen atom will not be ionized at physiological pH.
A. Consider the side chains of the. Assume pH=7.4.
**Pyridine (azine) nitrogen atom will not be ionized at physiological pH.
B. Determine which of the side chains are both at pH=7.4.
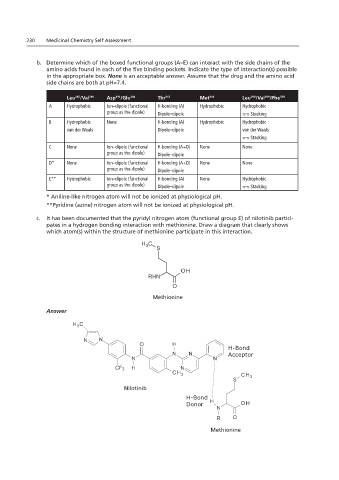
c. It has been documented that the pyridyl nitrogen atom (functional group E) of nilotinib partici-
pates in a hydrogen bonding interaction with methionine. Draw a diagram that clearly shows
C. It has been atom(s) within the structure of methionine participate in this interaction.
which atom(s) within the structure of methionine participate in this interaction.
Answer: Methionine
Methionine
Answer
H-Bond
Acceptor
Nilotinib
Nilotinib
H-Bond
Donor
Methionine
Methionine