Page 240 - Medicinal Chemistry Self Assessment
P. 240
2.26 Sorafenib 229
2.26 Sorafenib
Because protein tyrosine kinases owth is instrumental in the generation of new blood vessels.
Answer
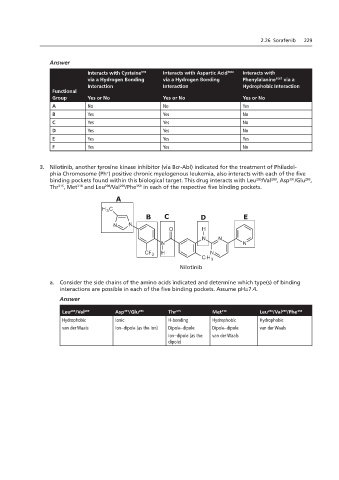
Interacts with Cysteine C Interacts with Aspartic Acid 1046 Interacts with
919
F
via a Hydrogen Bonding via a Hydrogen Bonding Phenylalanine 1047 via a
Interaction Interaction E Hydrophobic Interaction
Functional
Group Yes or No Yes or No Yes or No
A No A No Yes
B Yes Yes No
C Yes Yes No
D Yes B Yes D No
E Yes Yes Yes
F Yes Yes Sorafenib No
1. Conduct a to the questions that follow.
3. Nilotinib, another tyrosine kinase inhibitor (via Bcr-Abl) indicated for the treatment of Philadel-
2. Sorafenib interacts with in the local environment of the enzyme.
+
phia Chromosome (Ph ) positive chronic myelogenous leukemia, also interacts with each of the five
binding pockets found within this biological target. This drug interacts with Leu /Val , Asp /Glu ,
391
286
289
285
359
298
299
359 /Val
in each of the respective five binding pockets.
315 3. Nilotinib, another and Leu
/Phe
Thr , Met and Leu /Val /Phe in each of the respective five binding pockets.
299
298
318
A
B C D E
Nilotinib
Nilotinib
a. Consider the side chains of the amino acids indicated and determine which type(s) of binding
interactions are possible in each of the five binding pockets. Assume pH=7.4.
Answer
A. Consider the side chains of the. Assume pH=7.4.
Leu /Val 289 Asp /Glu 286 Thr 315 Met 318 Leu /Val /Phe 359
298
285
391
299
B. Determine which of the side chains are both at pH=7.4.
Hydrophobic Ionic H-bonding Hydrophobic Hydrophobic
C. It has been atom(s) within the structure of methionine participate in this interaction.
van der Waals Ion–dipole (as the ion) Dipole–dipole Dipole–dipole van der Waals
Ion–dipole (as the van der Waals
dipole)
Methionine