Page 235 - Medicinal Chemistry Self Assessment
P. 235
2.25 Sitagliptin
2.25 Sitagliptin
Sitagliptin is an inhibitor of dipeptidyl represents the amino-terminal alanine residue found within GLP-1.
A
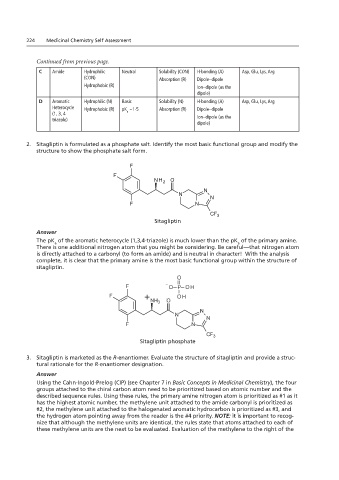
224 Medicinal Chemistry Self Assessment
Sitagliptin is an inhibitor of dipeptidyl represents the amino-terminal alanine residue found within GLP-1.
F B
F A C
Continued from previous page. F NH 2 O D
C
N
C Amide Hydrophilic F Neutral B Solubility (CON) H-bonding (A) Asp, Glu, Lys, Arg
N
O
N
(CON) NH Absorption (R) Dipole–dipole
D
2
Hydrophobic (R) F N Ion–dipole (as the
N
N dipole) CF 3
N
F
D Aromatic Hydrophilic (N) Basic Sitagliptin N H-bonding (A) Asp, Glu, Lys, Arg
Solubility (N)
Heterocycle Hydrophobic (R) pK ~1-5 Absorption (R) Dipole–dipole
(1, 3, 4 a CF 3
triazole) Sitagliptin Ion–dipole (as the
dipole)
1. Conduct a grid to inform the answers to the questions that follow.
2. Sitagliptin is group and modify the structure to show the phosphate salt form.
1. Conduct a grid to inform the answers to the questions that follow.
2. Sitagliptin is formulated as a phosphate salt. Identify the most basic functional group and modify the
structure to show the phosphate salt form.
2. Sitagliptin is group and modify the structure to show the phosphate salt form.
F
F
F NH 2 O
F N N
NH 2 O N
F N N
N
N CF 3
F Sitagliptin N
Sitagliptin
Answer CF 3
Answer:
Sitagliptin
The pK of the aromatic heterocycle (1,3,4-triazole) is much lower than the pK of the primary amine.
There is one additional nitrogen atom that you might be considering. Be careful—that nitrogen atom
a
a
The pK a the primary amine is the most basic functional group within the structure of sitagliptin.
Answer:
is directly attached to a carbonyl (to form an amide) and is neutral in character! With the analysis
The pK a the primary amine is the most basic functional group within the structure of sitagliptin.
complete, it is clear that the primary amine is the most basic functional group within the structure of
sitagliptin.
Sitagliptin phosphate
Sitagliptin phosphate
Sitagliptin phosphate
3. Sitagliptin is marketed as the R-enantiomer. Evaluate the structure of sitagliptin and provide a struc-
tural rationale for the R-enantiomer designation.
Answer
Using the Cahn-Ingold-Prelog (CIP) (see Chapter 7 in Basic Concepts in Medicinal Chemistry), the four
groups attached to the chiral carbon atom need to be prioritized based on atomic number and the
described sequence rules. Using these rules, the primary amine nitrogen atom is prioritized as #1 as it
has the highest atomic number, the methylene unit attached to the amide carbonyl is prioritized as
#2, the methylene unit attached to the halogenated aromatic hydrocarbon is prioritized as #3, and
the hydrogen atom pointing away from the reader is the #4 priority. NOTE: it is important to recog-
nize that although the methylene units are identical, the rules state that atoms attached to each of
these methylene units are the next to be evaluated. Evaluation of the methylene to the right of the