Page 245 - 00. Complete Version - Progress Report IPEN 2014-2016
P. 245
Environmental Science and Technology | Progress Report 245
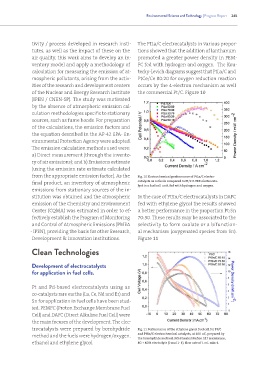
tivity / process developed in research insti- The PtLa/C electrocatalysts in various propor-
tutes, as well as the impact of these on the tions showed that the addition of lanthanum
air quality, this work aims to develop an in- promoted a greater power density in PEM-
ventory model and apply a methodology of FC fed with hydrogen and oxygen. The Kou-
calculation for measuring the emission of at- tecky-Levich diagrams suggest that PtLa/C and
mospheric pollutants, arising from the activ- PtCe/Ce 80:20 for oxygen reduction reaction
ities of the research and development centers occurs by the 4-electron mechanism as well
of the Nuclear and Energy Research Institute the commercial Pt/C. Figure 10
(IPEN / CNEN-SP). The study was motivated
by the absence of atmospheric emission cal-
culation methodologies specific to stationary
sources, such as fume hoods. For preparation
of the calculations, the emission factors and
the equation described in the AP-42 EPA- En-
vironmental Protection Agency were adopted.
The emission calculation methods used were:
a) Direct measurement (through the invento-
ry of air emissions); and b) Emissions estimate
(using the emission rate estimate calculated
from the appropriate emission factor). As the Fig. 10 Electrochemical performance of PtLa/C electro-
final product, an inventory of atmospheric catalysts as cathode compared to Pt/C E-TEK electrocata-
lyst in a fuel cell unit, fed with hydrogen and oxygen.
emissions from stationary sources of the in-
stitution was obtained and the atmospheric In the case of PtSn/C electrocatalysts in DAFC
emission of the Chemistry and Environment fed with ethylene glycol the results showed
Center (CQMA) was estimated in order to ef- a better performance in the proportion Pt:Sn
fectively establish the Program of Monitoring 70:30. These results may be associated to the
and Control of Atmospheric Emissions (PMEA selectivity to form oxalate or a bifunction-
- IPEN), providing the basis for other Research, al mechanism (oxygenated species from Sn).
Development & Innovation institutions. Figure 11
Clean Technologies
Development of electrocatalysts
for application in fuel cells.
Pt and Pd-based electrocatalysts using as
co-catalysts rare earths (La, Ce, Nd and Er) and
Sn for application in fuel cells have been stud-
ied. PEMFC (Proton Exchange Membrane Fuel
Cell) and DAFC (Direct Alkaline Fuel Cell) were
the main focuses of the development. The elec-
trocatalysts were prepared by borohydride Fig. 11 Performance of the ethylene glycol fuel cell for Pt/C
method and the fuels were hydrogen/oxygen, and PtSn/C electrochemical catalysts, at 100 oC, prepared by
the borohydride method, KOH treated Nafion 117 membrane,
ethanol and ethylene glycol. EG + KOH electrolyte (2 mol L- 1), flow rate of 1 mL min-1.