Page 7 - Extemp Marking Scheme
P. 7
FAR 222/3 Dosage Form II Extemporaneous Exercise – Marking Scheme 2019 – 2020
Exercise 3.5 A
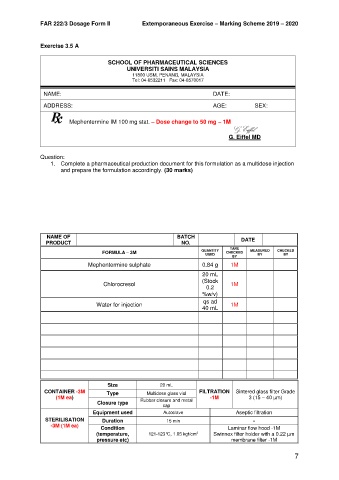
SCHOOL OF PHARMACEUTICAL SCIENCES
UNIVERSITI SAINS MALAYSIA
11800 USM, PENANG, MALAYSIA
Tel: 04-6532211 Fax: 04-6570017
NAME: DATE:
ADDRESS: AGE: SEX:
Mephentermine IM 100 mg stat. – Dose change to 50 mg – 1M
G.Eiffel
G. Eiffel MD
Question:
1. Complete a pharmaceutical production document for this formulation as a multidose injection
and prepare the formulation accordingly. (30 marks)
NAME OF BATCH
PRODUCT NO. DATE
TARE MEASURED CHECKED
QUANTITY
FORMULA – 3M USED CHECKED BY BY
BY
Mephentermine sulphate 0.84 g 1M
20 mL
(Stock
Chlorocresol 1M
0.2
%w/v)
qs ad
Water for injection 1M
40 mL
Size 20 mL
CONTAINER -3M Type Multidose glass vial FILTRATION Sintered glass filter Grade
(1M ea) Rubber closure and metal -1M 3 (15 – 40 µm)
Closure type cap
Equipment used Autoclave Aseptic filtration
STERILISATION Duration 15 min -
-3M (1M ea) Condition Laminar flow hood -1M
(temperature, 121-123°C, 1.05 kgf/cm Swinnex filter holder with a 0.22 µm
2
pressure etc) membrane filter -1M
7