Page 263 - Natural Antioxidants, Applications in Foods of Animal Origin
P. 263
242 Natural Antioxidants: Applications in Foods of Animal Origin
VetBooks.ir Meat samples are prepared by first comminuting meat in a 38 °F (3 °C)
room, the appropriate solvent is added to the meat to convert mixture into a
form compatible for the analysis. Controlled heating volatilizes the extracted
compounds prior to injection via an inert gas onto the column. The column
can be thought of as consisting of a structural support (e.g., stainless steel)
lined with a stable film (stationary/liquid phase) that separates the complex
mixture of volatiles and secondary oxidation products in the meat sample.
Separation is best achieved when the polarity of the stationary phase is
similar in polarity to the compounds being analyzed.
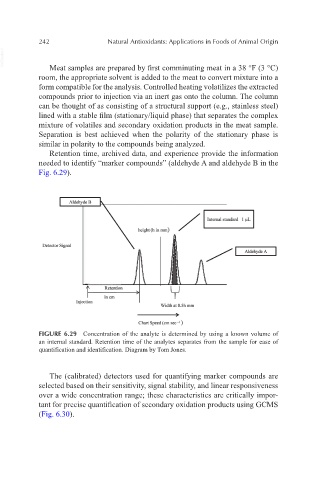
Retention time, archived data, and experience provide the information
needed to identify “marker compounds” (aldehyde A and aldehyde B in the
Fig. 6.29).
Aldehyde B
/ L..l-n-te_rn_a_l_s_tan-dar_d_l_ll_L_,
height (h in mm)
Detector Signal
/ LI_A_l_de_h.;..yd_e_A _ _.
Retention
in em
Injection
Width at 0.5h mm
Chart Speed (em sec -I)
FIGURE 6.29 Concentration of the analyte is determined by using a known volume of
an internal standard. Retention time of the analytes separates from the sample for ease of
quantification and identification. Diagram by Tom Jones.
The (calibrated) detectors used for quantifying marker compounds are
selected based on their sensitivity, signal stability, and linear responsiveness
over a wide concentration range; these characteristics are critically impor-
tant for precise quantification of secondary oxidation products using GCMS
(Fig. 6.30).