Page 642 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 642
628 SECTION VI Drugs Used to Treat Diseases of the Blood, Inflammation, & Gout
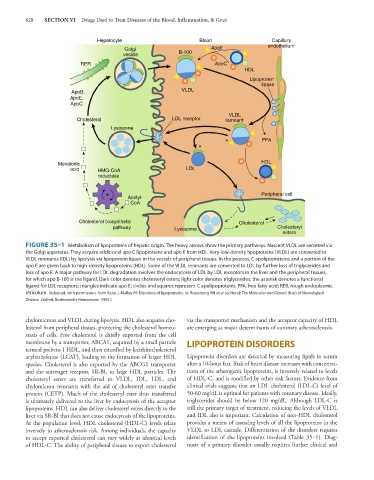
Hepatocyte Blood Capillary
Golgi ApoE endothelium
vesicle B-100
RER ApoC
HDL
Lipoprotein
lipase
ApoB, VLDL
ApoE,
ApoC
VLDL
Cholesterol LDL receptor remnant
Lysosome
* FFA
*
Mevalonic HDL
acid HMG-CoA LDL
reductase
Peripheral cell
Acetyl-
CoA
Cholesterol biosynthetic Cholesterol
pathway Lysosome Cholesteryl
esters
FIGURE 35–1 Metabolism of lipoproteins of hepatic origin. The heavy arrows show the primary pathways. Nascent VLDL are secreted via
the Golgi apparatus. They acquire additional apo C lipoproteins and apo E from HDL. Very-low-density lipoproteins (VLDL) are converted to
VLDL remnants (IDL) by lipolysis via lipoprotein lipase in the vessels of peripheral tissues. In the process, C apolipoproteins and a portion of the
apo E are given back to high-density lipoproteins (HDL). Some of the VLDL remnants are converted to LDL by further loss of triglycerides and
loss of apo E. A major pathway for LDL degradation involves the endocytosis of LDL by LDL receptors in the liver and the peripheral tissues,
for which apo B-100 is the ligand. Dark color denotes cholesteryl esters; light color denotes triglycerides; the asterisk denotes a functional
ligand for LDL receptors; triangles indicate apo E; circles and squares represent C apolipoproteins. FFA, free fatty acid; RER, rough endoplasmic
reticulum. (Adapted, with permission, from Kane J, Malloy M: Disorders of lipoproteins. In: Rosenberg RN et al [editors]: The Molecular and Genetic Basis of Neurological
Disease. 2nd ed. Butterworth-Heinemann, 1997.)
chylomicrons and VLDL during lipolysis. HDL also acquires cho- via the transporter mechanism and the acceptor capacity of HDL
lesterol from peripheral tissues, protecting the cholesterol homeo- are emerging as major determinants of coronary atherosclerosis.
stasis of cells. Free cholesterol is chiefly exported from the cell
membrane by a transporter, ABCA1, acquired by a small particle LIPOPROTEIN DISORDERS
termed prebeta-1 HDL, and then esterified by lecithin:cholesterol
acyltransferase (LCAT), leading to the formation of larger HDL Lipoprotein disorders are detected by measuring lipids in serum
species. Cholesterol is also exported by the ABCG1 transporter after a 10-hour fast. Risk of heart disease increases with concentra-
and the scavenger receptor, SR-BI, to large HDL particles. The tions of the atherogenic lipoproteins, is inversely related to levels
cholesteryl esters are transferred to VLDL, IDL, LDL, and of HDL-C, and is modified by other risk factors. Evidence from
chylomicron remnants with the aid of cholesteryl ester transfer clinical trials suggests that an LDL cholesterol (LDL-C) level of
protein (CETP). Much of the cholesteryl ester thus transferred 50-60 mg/dL is optimal for patients with coronary disease. Ideally,
is ultimately delivered to the liver by endocytosis of the acceptor triglycerides should be below 120 mg/dL. Although LDL-C is
lipoproteins. HDL can also deliver cholesteryl esters directly to the still the primary target of treatment, reducing the levels of VLDL
liver via SR-BI that does not cause endocytosis of the lipoproteins. and IDL also is important. Calculation of non-HDL cholesterol
At the population level, HDL cholesterol (HDL-C) levels relate provides a means of assessing levels of all the lipoproteins in the
inversely to atherosclerosis risk. Among individuals, the capacity VLDL to LDL cascade. Differentiation of the disorders requires
to accept exported cholesterol can vary widely at identical levels identification of the lipoproteins involved (Table 35–1). Diag-
of HDL-C. The ability of peripheral tissues to export cholesterol nosis of a primary disorder usually requires further clinical and