Page 6 - Parker - Bottled water filtration
P. 6
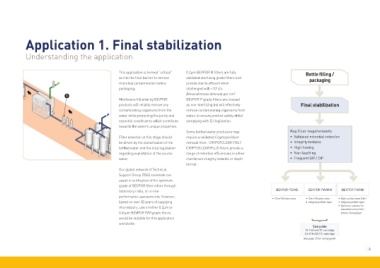
Application 1. Final stabilization
Understanding the application
This application is termed “critical” 0.2µm BEVPOR M filters are fully Bottle filling /
as it is the final barrier to remove validated sterilizing grade filters and packaging
microbial contamination before provide sterile effluent when
packaging. challenged with >10 cfu
7
➊ Brevundimonas diminuta per cm . 2
Membrane filtration by BEVPOR BEVPOR P grade filters are classed
products will reliably remove any as non-sterilizing but will effectively Final stabilization
contaminating organisms from the remove contaminating organisms from
water while protecting the purity and water, to ensure product safety whilst
essential constituents which contribute complying with EU legislation.
towards the water’s unique properties.
Some bottled water producers may Key filter requirements
Filter selection at this stage should require a validated Cryptosporidium • Validated microbial retention
be driven by the classification of the removal filter. CRYPOTCLEAR PES / • Integrity testable
bottled water and the local legislation CRYPTOCLEAR PLUS filters provide a • High flowing
regarding exploitation of the source range of retention efficiencies in either • Non-leaching
water. membrane integrity testable or depth • Frequent SIP / CIP
format.
Our global network of Technical
Support Group (TSG) scientists can
assist in verification of the optimum
grade of BEVPOR filter either through
laboratory trials, or in-line BEVPOR PS/MS BEVPOR PW/MW BEVPOR PH/MH
performance assessments. However, • 0.6m 2 filtration area • 0.6m 2 filtration area • High surface area 0.8m 2
based on over 30 years of supplying • Integral prefilter layer • Integral prefilter layer
this industry, use of either 0.2µm or • Optimum solution for
extended service life /
0.45µm BEVPOR P/M grade filters volume throughput
would be suitable for this application
worldwide. }
Size guide
10-15l/min/10¨cartridge
0.6-0.9m 3 /h/10¨cartridge
See page 15 for rating guide
| 6