Page 42 - C:\Users\MY PC\Desktop\FlipBook
P. 42
44
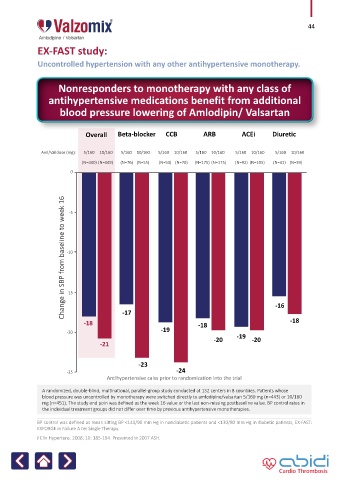
EX-FAST study:
Uncontrolled hypertension with any other antihypertensive monotherapy.
Nonresponders to monotherapy with any class of
antihypertensive medications benefit from additional
blood pressure lowering of Amlodipin/ Valsartan
Overall Beta-blocker CCB ARB ACEi Diuretic
Aml/Val dose (mg): 5/160 10/160 5/160 10/160 5/160 10/160 5/160 10/160 5/160 10/160 5/160 10/160
(N=440) (N=449) (N=76) (N=55) (N=53) (N=70) (N=175) (N=175) (N=92) (N=105) (N=41) (N=39)
0
Change in SBP from baseline to week 16
-5
-10
-15
-16
-17
-18 -18 -18
-20 -19 -19
-20 -20
-21
-23
-25 -24
Antihypertensive calss prior to randomization into the trial
A randomized, double-blind, multinational, parallel-group study conducted at 132 centers in 8 countries. Patients whose
blood pressure was uncontrolled by monotherapy were switched directly to amlodipine/valsartan 5/160 mg (n=443) or 10/160
mg (n=451). The study end poin was defined as the week 16 value or the last non-missing postbaseline value. BP control rates in
the individual treatment groups did not differ over time by previous antihypertensive monotherapies.
BP control was defined as mean sitting BP <140/90 mm Hg in nondiabetic patients and <130/80 mm Hg in diabetic patients, EX-FAST:
EXFORGE in Failure A ter Single Therapy.
J Clin Hypertens. 2008; 10: 185-194. Presented in 2007 ASH.