Page 44 - C:\Users\MY PC\Desktop\FlipBook
P. 44
46
Amlodipine/ Valsartan combination has favorable
safety profile
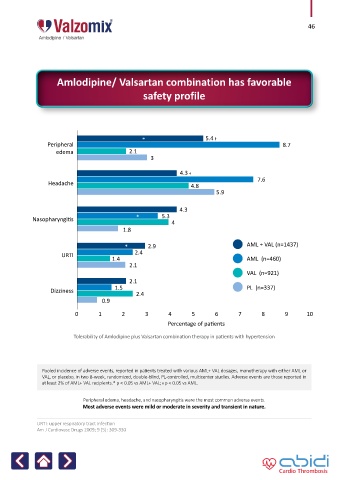
* 5.4 ᵻ
Peripheral 8.7
edema 2.1
3
4.3 ᵻ
7.6
Headache 4.8
5.9
4.3
Nasopharyngitis * 5.3 4
1.8
* 2.9 AML + VAL (n=1437)
URTI 2.4
1.4 AML (n=460)
2.1
VAL (n=921)
2.1
Dizziness 1.5 PL (n=337)
2.4
0.9
0 1 2 3 4 5 6 7 8 9 10
Percentage of patients
Tolerability of Amlodipine plus Valsartan combination therapy in patients with hypertension
Pooled incidence of adverse events, reported in patients treated with various AML+ VAL dosages, monotherapy with either AML or
VAL, or placebo, in two 8-week, randomized, double-blind, PL-controlled, multicenter studies. Adverse events are those reported in
at least 2% of AML+ VAL recipients.* p < 0.05 vs AML+ VAL; ᵻ p < 0.05 vs AML.
Peripheral edema, headache, and nasopharyngitis were the most common adverse events.
Most adverse events were mild or moderate in severity and transient in nature.
URTI: upper respiratory tract infection
Am J Cardiovasc Drugs 2009; 9 (5): 309-330