Page 15 - Analytical Chemistry I E-book
P. 15
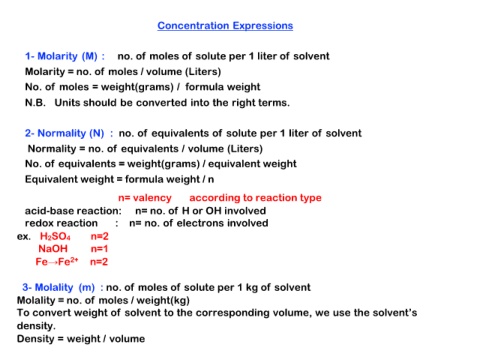
Concentration Expressions
1- Molarity (M) : no. of moles of solute per 1 liter of solvent
Molarity = no. of moles / volume (Liters)
No. of moles = weight(grams) / formula weight
N.B. Units should be converted into the right terms.
2- Normality (N) : no. of equivalents of solute per 1 liter of solvent
Normality = no. of equivalents / volume (Liters)
No. of equivalents = weight(grams) / equivalent weight
Equivalent weight = formula weight / n
n= valency according to reaction type
acid-base reaction: n= no. of H or OH involved
redox reaction : n= no. of electrons involved
ex. H2SO4 n=2
NaOH n=1
Fe→Fe2+ n=2
3- Molality (m) : no. of moles of solute per 1 kg of solvent
Molality = no. of moles / weight(kg)
To convert weight of solvent to the corresponding volume, we use the solvent’s
density.
Density = weight / volume