Page 19 - Analytical Chemistry I E-book
P. 19
3-Net ionic equation
is an ionic equation from which spectator ions have been cancelled. A spectator
ion is an ion in an ionic equation that does not take part in the reaction. In the
above example, Na+ and CO32- ions appear on both sides of the equation. This
means that nothing happens to these ions as the reaction occurs, so they are
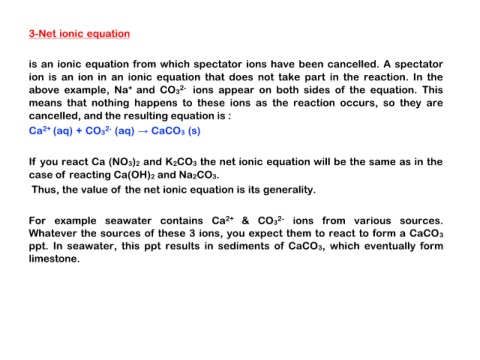
cancelled, and the resulting equation is :
Ca2+ (aq) + CO32- (aq) → CaCO3 (s)
If you react Ca (NO3)2 and K2CO3 the net ionic equation will be the same as in the
case of reacting Ca(OH)2 and Na2CO3.
Thus, the value of the net ionic equation is its generality.
For example seawater contains Ca2+ & CO32- ions from various sources.
Whatever the sources of these 3 ions, you expect them to react to form a CaCO3
ppt. In seawater, this ppt results in sediments of CaCO3, which eventually form
limestone.