Page 21 - Analytical Chemistry I E-book
P. 21
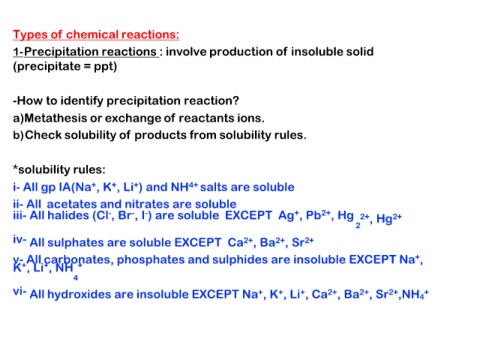
Types of chemical reactions:
1-Precipitation reactions : involve production of insoluble solid
(precipitate = ppt)
-How to identify precipitation reaction?
a)Metathesis or exchange of reactants ions.
b)Check solubility of products from solubility rules.
*solubility rules:
i- All gp IA(Na+, K+, Li+) and NH4+ salts are soluble
ii- All acetates and nitrates are soluble
iii- All halides (Cl-, Br-, I-) are soluble EXCEPT Ag+, Pb2+, Hg 22+, Hg2+
iv- All sulphates are soluble EXCEPT Ca2+, Ba2+, Sr2+
vK-+A, Lllic+,aNrbHo+nates, phosphates and sulphides are insoluble EXCEPT Na+,
4
vi- All hydroxides are insoluble EXCEPT Na+, K+, Li+, Ca2+, Ba2+, Sr2+,NH4+