Page 23 - Analytical Chemistry I E-book
P. 23
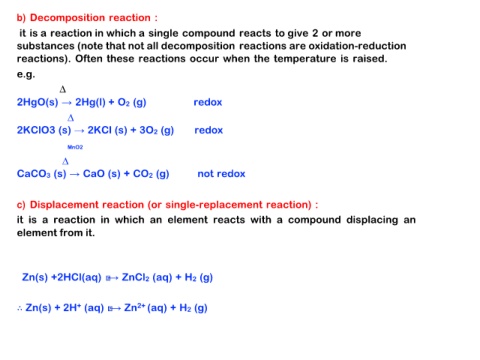
b) Decomposition reaction :
it is a reaction in which a single compound reacts to give 2 or more
substances (note that not all decomposition reactions are oxidation-reduction
reactions). Often these reactions occur when the temperature is raised.
e.g.
∆ redox
2HgO(s) → 2Hg(l) + O2 (g)
∆ redox
2KClO3 (s) → 2KCl (s) + 3O2 (g)
MnO2 not redox
∆
CaCO3 (s) → CaO (s) + CO2 (g)
c) Displacement reaction (or single-replacement reaction) :
it is a reaction in which an element reacts with a compound displacing an
element from it.
Zn(s) +2HCl(aq) → ZnCl2 (aq) + H2 (g)
∴ Zn(s) + 2H+ (aq) → Zn2+ (aq) + H2 (g)