Page 22 - Analytical Chemistry I E-book
P. 22
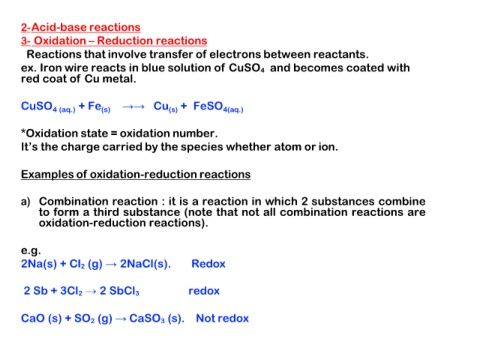
2-Acid-base reactions
3- Oxidation – Reduction reactions
Reactions that involve transfer of electrons between reactants.
ex. Iron wire reacts in blue solution of CuSO4 and becomes coated with
red coat of Cu metal.
CuSO4 (aq.) + Fe(s) →→ Cu(s) + FeSO4(aq.)
*Oxidation state = oxidation number.
It’s the charge carried by the species whether atom or ion.
Examples of oxidation-reduction reactions
a) Combination reaction : it is a reaction in which 2 substances combine
to form a third substance (note that not all combination reactions are
oxidation-reduction reactions).
e.g.
2Na(s) + Cl2 (g) → 2NaCl(s). Redox
2 Sb + 3Cl2 → 2 SbCl3 redox
CaO (s) + SO2 (g) → CaSO3 (s). Not redox