Page 20 - Analytical Chemistry I E-book
P. 20
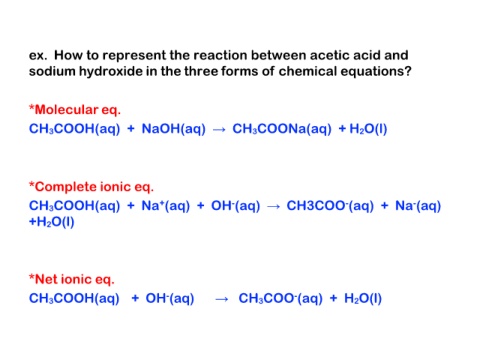
ex. How to represent the reaction between acetic acid and
sodium hydroxide in the three forms of chemical equations?
*Molecular eq.
CH3COOH(aq) + NaOH(aq) → CH3COONa(aq) + H2O(l)
*Complete ionic eq.
CH3COOH(aq) + Na+(aq) + OH-(aq) → CH3COO-(aq) + Na-(aq)
+H2O(l)
*Net ionic eq.
CH3COOH(aq) + OH-(aq) → CH3COO-(aq) + H2O(l)