Page 16 - Analytical Chemistry I E-book
P. 16
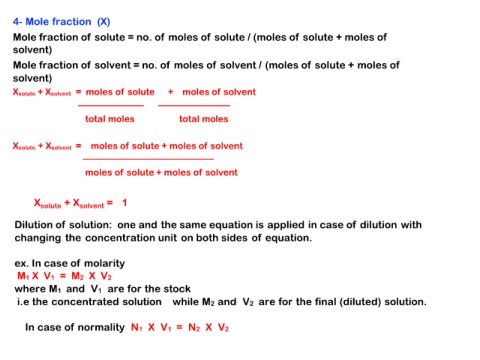
4- Mole fraction (X)
Mole fraction of solute = no. of moles of solute / (moles of solute + moles of
solvent)
Mole fraction of solvent = no. of moles of solvent / (moles of solute + moles of
solvent)
Xsolute + Xsolvent = moles of solute + moles of solvent
────────── ───────────
total moles total moles
Xsolute + Xsolvent = moles of solute + moles of solvent
────────────────────
moles of solute + moles of solvent
Xsolute + Xsolvent = 1
Dilution of solution: one and the same equation is applied in case of dilution with
changing the concentration unit on both sides of equation.
ex. In case of molarity
M1 X V1 = M2 X V2
where M1 and V1 are for the stock
i.e the concentrated solution while M2 and V2 are for the final (diluted) solution.
In case of normality N1 X V1 = N2 X V2