Page 18 - Analytical Chemistry I E-book
P. 18
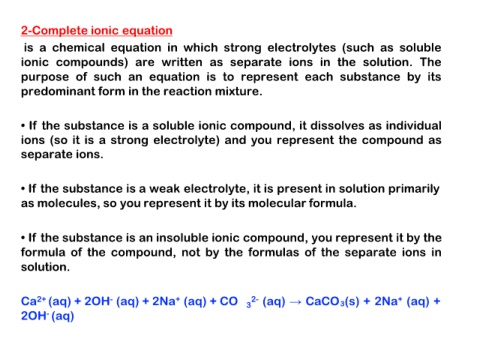
2-Complete ionic equation
is a chemical equation in which strong electrolytes (such as soluble
ionic compounds) are written as separate ions in the solution. The
purpose of such an equation is to represent each substance by its
predominant form in the reaction mixture.
• If the substance is a soluble ionic compound, it dissolves as individual
ions (so it is a strong electrolyte) and you represent the compound as
separate ions.
• If the substance is a weak electrolyte, it is present in solution primarily
as molecules, so you represent it by its molecular formula.
• If the substance is an insoluble ionic compound, you represent it by the
formula of the compound, not by the formulas of the separate ions in
solution.
Ca2+ (aq) + 2OH- (aq) + 2Na+ (aq) + CO 32- (aq) → CaCO3(s) + 2Na+ (aq) +
2OH- (aq)