Page 61 - Analytical Chemistry I E-book
P. 61
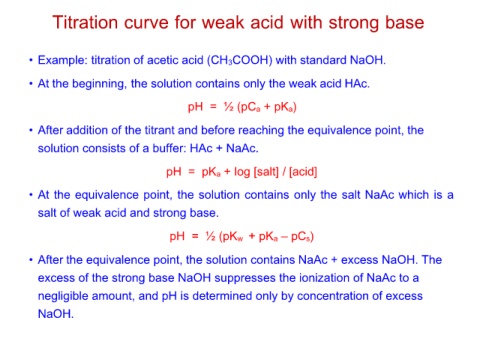
Titration curve for weak acid with strong base
• Example: titration of acetic acid (CH3COOH) with standard NaOH.
• At the beginning, the solution contains only the weak acid HAc.
pH = ½ (pCa + pKa)
• After addition of the titrant and before reaching the equivalence point, the
solution consists of a buffer: HAc + NaAc.
pH = pKa + log [salt] / [acid]
• At the equivalence point, the solution contains only the salt NaAc which is a
salt of weak acid and strong base.
pH = ½ (pKw + pKa – pCs)
• After the equivalence point, the solution contains NaAc + excess NaOH. The
excess of the strong base NaOH suppresses the ionization of NaAc to a
negligible amount, and pH is determined only by concentration of excess
NaOH.