Page 62 - Analytical Chemistry I E-book
P. 62
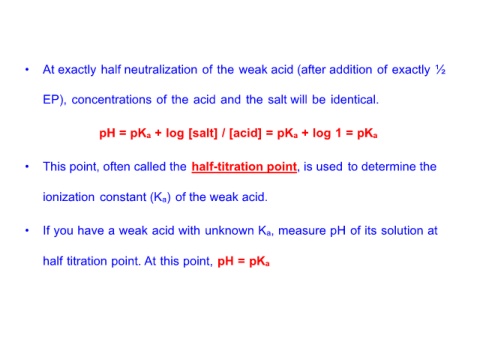
• At exactly half neutralization of the weak acid (after addition of exactly ½
EP), concentrations of the acid and the salt will be identical.
pH = pKa + log [salt] / [acid] = pKa + log 1 = pKa
• This point, often called the half-titration point, is used to determine the
ionization constant (Ka) of the weak acid.
• If you have a weak acid with unknown Ka, measure pH of its solution at
half titration point. At this point, pH = pKa