Page 65 - Analytical Chemistry I E-book
P. 65
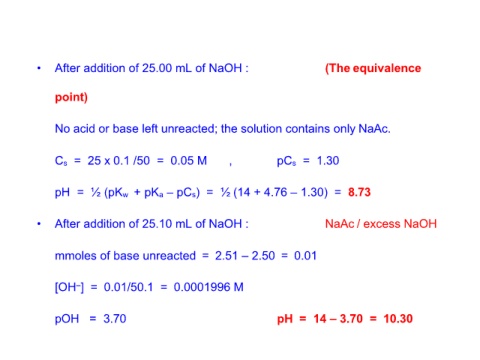
• After addition of 25.00 mL of NaOH : (The equivalence
point)
No acid or base left unreacted; the solution contains only NaAc.
Cs = 25 x 0.1 /50 = 0.05 M , pCs = 1.30
pH = ½ (pKw + pKa – pCs) = ½ (14 + 4.76 – 1.30) = 8.73
• After addition of 25.10 mL of NaOH : NaAc / excess NaOH
mmoles of base unreacted = 2.51 – 2.50 = 0.01
[OH–] = 0.01/50.1 = 0.0001996 M
pOH = 3.70 pH = 14 – 3.70 = 10.30