Page 64 - Analytical Chemistry I E-book
P. 64
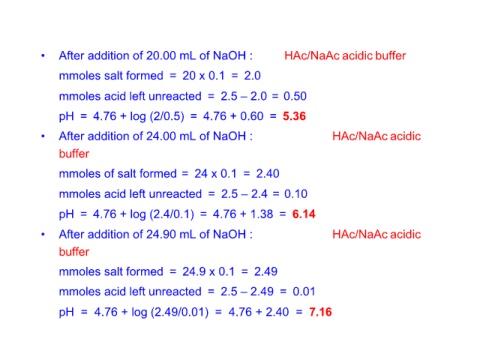
• After addition of 20.00 mL of NaOH : HAc/NaAc acidic buffer
mmoles salt formed = 20 x 0.1 = 2.0
mmoles acid left unreacted = 2.5 – 2.0 = 0.50
pH = 4.76 + log (2/0.5) = 4.76 + 0.60 = 5.36
• After addition of 24.00 mL of NaOH : HAc/NaAc acidic
buffer
mmoles of salt formed = 24 x 0.1 = 2.40
mmoles acid left unreacted = 2.5 – 2.4 = 0.10
pH = 4.76 + log (2.4/0.1) = 4.76 + 1.38 = 6.14
• After addition of 24.90 mL of NaOH : HAc/NaAc acidic
buffer
mmoles salt formed = 24.9 x 0.1 = 2.49
mmoles acid left unreacted = 2.5 – 2.49 = 0.01
pH = 4.76 + log (2.49/0.01) = 4.76 + 2.40 = 7.16