Page 68 - Analytical Chemistry I E-book
P. 68
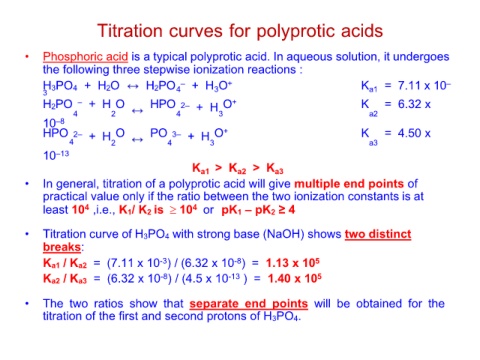
Titration curves for polyprotic acids
• Phosphoric acid is a typical polyprotic acid. In aqueous solution, it undergoes
the following three stepwise ionization reactions :
H3 3PO4 + H2O H2PO4– + H3O+ Ka1 = 7.11 x 10–
H2PO – + H O HPO 2– + H3O+ K = 6.32 x
42
10–8 4 a2
HPO 2– + H2O PO 3– + H3O+ K = 4.50 x
4 4 a3
10–13 Ka1 > Ka2 > Ka3
• In general, titration of a polyprotic acid will give multiple end points of
practical value only if the ratio between the two ionization constants is at
least 104 ,i.e., K1/ K2 is 104 or pK1 – pK2 ≥ 4
• Titration curve of H3PO4 with strong base (NaOH) shows two distinct
breaks:
Ka1 / Ka2 = (7.11 x 10-3) / (6.32 x 10-8) = 1.13 x 105
Ka2 / Ka3 = (6.32 x 10-8) / (4.5 x 10-13 ) = 1.40 x 105
• The two ratios show that separate end points will be obtained for the
titration of the first and second protons of H3PO4.