Page 72 - Analytical Chemistry I E-book
P. 72
Feasibility of Titrations
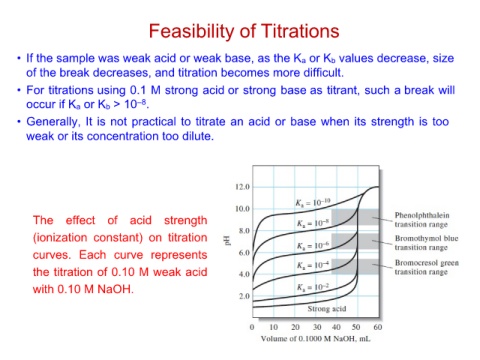
• If the sample was weak acid or weak base, as the Ka or Kb values decrease, size
of the break decreases, and titration becomes more difficult.
• For titrations using 0.1 M strong acid or strong base as titrant, such a break will
occur if Ka or Kb > 10–8.
• Generally, It is not practical to titrate an acid or base when its strength is too
weak or its concentration too dilute.
The effect of acid strength
(ionization constant) on titration
curves. Each curve represents
the titration of 0.10 M weak acid
with 0.10 M NaOH.