Page 71 - Analytical Chemistry I E-book
P. 71
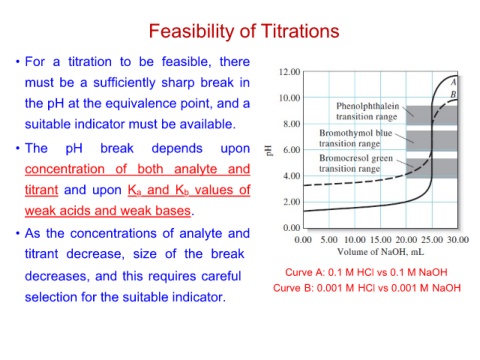
Feasibility of Titrations
• For a titration to be feasible, there Curve A: 0.1 M HCl vs 0.1 M NaOH
must be a sufficiently sharp break in Curve B: 0.001 M HCl vs 0.001 M NaOH
the pH at the equivalence point, and a
suitable indicator must be available.
• The pH break depends upon
concentration of both analyte and
titrant and upon Ka and Kb values of
weak acids and weak bases.
• As the concentrations of analyte and
titrant decrease, size of the break
decreases, and this requires careful
selection for the suitable indicator.