Page 63 - Analytical Chemistry I E-book
P. 63
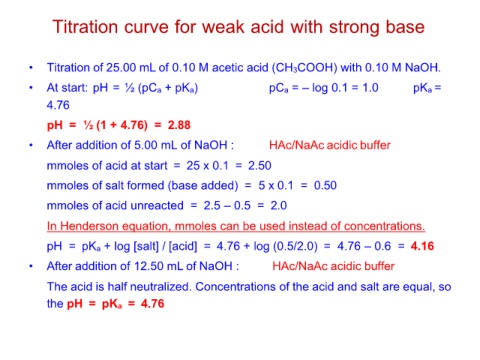
Titration curve for weak acid with strong base
• Titration of 25.00 mL of 0.10 M acetic acid (CH3COOH) with 0.10 M NaOH.
• At start: pH = ½ (pCa + pKa) pCa = – log 0.1 = 1.0 pKa =
4.76
pH = ½ (1 + 4.76) = 2.88
• After addition of 5.00 mL of NaOH : HAc/NaAc acidic buffer
mmoles of acid at start = 25 x 0.1 = 2.50
mmoles of salt formed (base added) = 5 x 0.1 = 0.50
mmoles of acid unreacted = 2.5 – 0.5 = 2.0
In Henderson equation, mmoles can be used instead of concentrations.
pH = pKa + log [salt] / [acid] = 4.76 + log (0.5/2.0) = 4.76 – 0.6 = 4.16
• After addition of 12.50 mL of NaOH : HAc/NaAc acidic buffer
The acid is half neutralized. Concentrations of the acid and salt are equal, so
the pH = pKa = 4.76