Page 32 - Practical DF2 Corrected (2)
P. 32
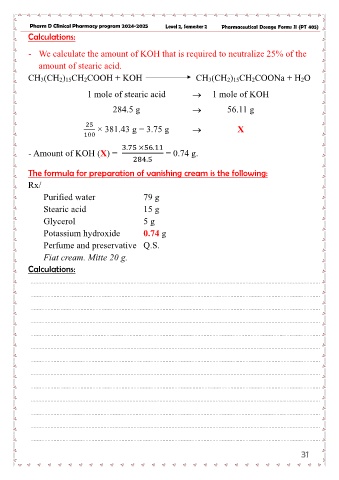
Pharm D Clinical Pharmacy program 2024-2025 Level 2, Semester 2 Pharmaceutical Dosage Forms II (PT 405)
Calculations:
- We calculate the amount of KOH that is required to neutralize 25% of the
amount of stearic acid.
CH3(CH2)15CH2COOH + KOH CH3(CH2)15CH2COONa + H2O
1 mole of stearic acid → 1 mole of KOH
284.5 g → 56.11 g
25 × 381.43 g = 3.75 g → X
100
- Amount of KOH (X) = 3.75 ×56.11 = 0.74 g.
284.5
The formula for preparation of vanishing cream is the following:
Rx/
Purified water 79 g
Stearic acid 15 g
Glycerol 5g
Potassium hydroxide 0.74 g
Perfume and preservative Q.S.
Fiat cream. Mitte 20 g.
Calculations:
………………………………………….…………………………………………………………………………….………………………………………………
…………………………….…………………………………………………………………………….……………………………………………………………
……………….…………………………………………………………………………….…………………………………………………………………………
……………….…………………………………………………………………………….…………………………………………………………………………
…………………………………………………………………….…………………………………………………………………………….……………………
……………………………………………………….…………………………………………………………………………….…………………………………
………………………………………….…………………………………………………………………………….………………………………………………
…………………………….…………………………………………………………………………….……………………………………………………………
……………….…………………………………………………………………………….…………………………………………………………………………
…………………………………………………………………….…………………………………………………………………………….……………………
…………………………………………………………………….…………………………………………………………………………….……………………
……………………………………………………….…………………………………………………………………………….…………………………………
………………………………………….…………………………………………………………………………….………………………………………………
31