Page 19 - 2- WEKI Center - الايزو جزء الشرح
P. 19
- ()
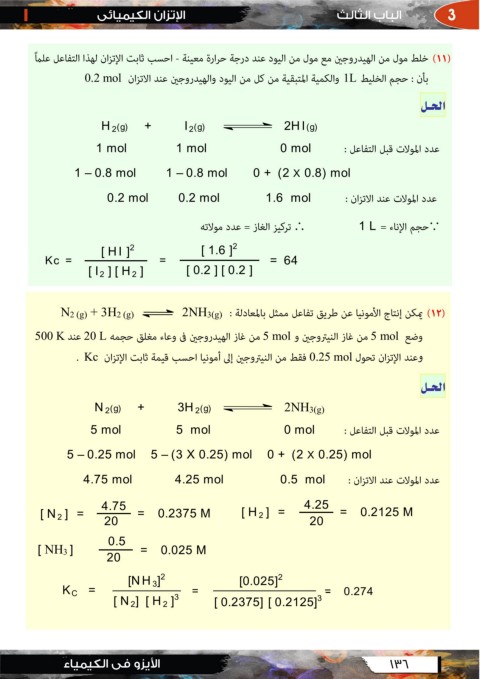
ﺍﻟﺤــﻞ
0.2 mol 1L
H2(g) + I2(g) 2HI(g)
1 mol 1 mol 0 mol
0 + (2 X 0.8) mol
1 – 0.8 mol 1 – 0.8 mol
0.2 mol 0.2 mol 1.6 mol
1L
Kc = [ HI ]2 = [ 1.6 ]2 = 64
[ 0.2 ] [ 0.2 ]
[ I2 ] [ H2 ]
N2 (g) + 3H2 (g) 2NH3(g) ()
500 K 20 L
5 mol 5 mol و
. Kc
0.25 mol
ﺍﻟﺤــﻞ
N2(g) + 3H2(g) 2NH3(g)
5 mol 5 mol 0 mol
5 – 0.25 mol 5 – (3 X 0.25) mol 0 + (2 X 0.25) mol
4.75 mol 4.25 mol 0.5 mol
[ N2 ] = 4.75 = 0.2375 M [ H2 ] = 4.25 = 0.2125 M
20 20
[ NH3 ] 0.5
= 20 = 0.025 M
KC = [NH3]2 = [0.025]2 0.274
[ N2] [ H2 ]3
=
[ 0.2375] [ 0.2125]3
١٣٥
136