Page 20 - 2- WEKI Center - الايزو جزء الشرح
P. 20
ﺇﻧﺘﺒـــﻪ :ﻳﺘﻢ ﺣﺴﺎﺏ ﻗﻴﻤﺔ ﺍﻟﺘﻐﻴﺮ ﻓﻰ ﺍﻟﻤﺤﺘﻮﻯ ﺍﻟﺤﺮﺍﺭﻯ ﻟﻠﺘﻔﺎﻋﻞ ∆Hﺑﺄﺣﺪ ﺍﻟﻘﺎﻧﻮﻧﻴﻦ ﺍﻵﺗﻴﻴﻦ .
= ∆Hﺎﻗﺔ اﻟ اﺗﺞ – ﺎﻗﺔ اﻟ ﻔﺎﻋﻼت .
= ∆Hﺎﻗﺔ ﺗ اﻟ ﻔﺎﻋﻞ اﻟ د – ﺎﻗﺔ ﺗ اﻟ ﻔﺎﻋﻞ اﻟﻌ ﻰ .
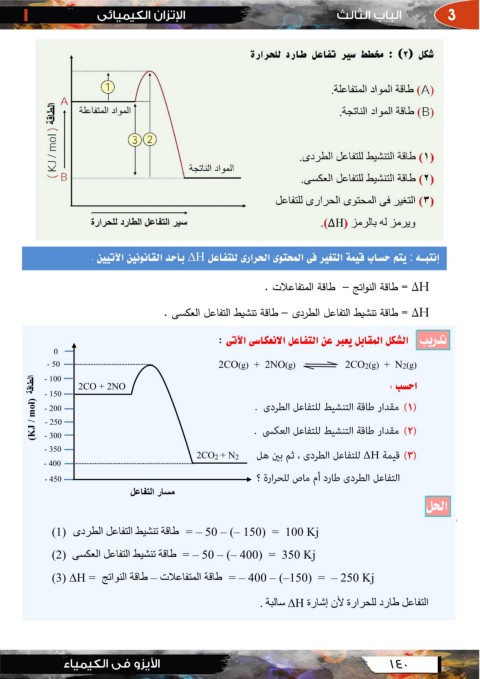
0 ﺍﻟﺸﻜﻞ ﺍﻟﻤﻘﺎﺑﻞ ﻳﻌﺒﺮ ﻋﻦ ﺍﻟﺘﻔﺎﻋﻞ ﺍﻻﻧﻌﻜﺎﺳﻰ ﺍﻵﺗﻰ :
- 50
اﻟﻄﺎﻗﺔ )(KJ / mol - 100 2CO + 2NO )2CO(g) + 2NO(g )2CO2(g) + N2(g
- 150
- 200 . ﺍﺣﺴﺐ :
- 250
- 300 )(
- 350
- 400 )( .
2CO2 + N2 )(
- 450
ﻣﺴﺎر اﻟﺘﻔﺎﻋﻞ
\
= – 50 – (– 150) = 100 Kjطﺎﻗﺔ ﺗﻨﺸﯿﻂ اﻟﺘﻔﺎﻋﻞ اﻟﻄﺮدى )(1
= – 50 – (– 400) = 350 Kjطﺎﻗﺔ ﺗﻨﺸﯿﻂ اﻟﺘﻔﺎﻋﻞ اﻟﻌﻜﺴﻰ )(2
= – 400 – (–150) = – 250 Kjطﺎﻗﺔ اﻟﻤﺘﻔﺎﻋﻼت – طﺎﻗﺔ اﻟﻨﻮاﺗﺞ = (3) ∆H
اﻟﺘﻔﺎﻋﻞ طﺎرد ﻟﻠﺤﺮارة ﻷن إﺷﺎرة Hﺳﺎﻟﺒﺔ .
١٣٩
140