Page 18 - 2- WEKI Center - الايزو جزء الشرح
P. 18
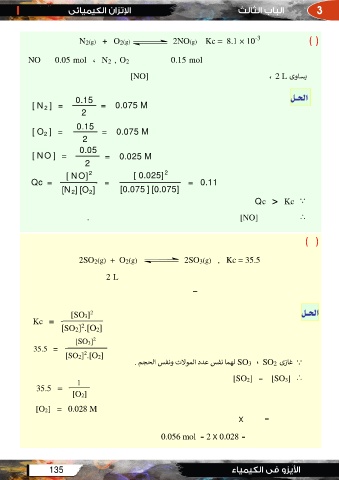
N2(g) + O2(g) 2NO(g) Kc = 8.1 × 10-3 ()
NO 0.05 mol ، N2 , O2 0.15 mol
[NO]
، 2 L ﺴﺎوى
[ N2 ] = 0.15 = 0.075 M ﺍﻟﺤــﻞ
2
[ O2 ] = 0.15 = 0.075 M
2
[ NO ] = 0.05
= 0.025 M
2
Qc = [ NO]2 = [ 0.025]2 = 0.11
[N2] [O2] [0.075 ] [0.075]
Qc > Kc
. [NO]
()
2SO2(g) + O2(g) 2SO3(g) , Kc = 35.5
2L
–
[SO3]2 ﺍﻟﺤــﻞ
Kc = [SO2]2.[O2]
. ﻟﻬﻤﺎ ﻧﻔﺲ ﻋﺪد اﻟﻤﻮﻻت وﻧﻔﺲ اﻟﺤﺠﻢSO3 ، SO2 ﻏﺎزى
[SO3]2
35.5 = [SO2] = [SO3]
[SO2]2.[O2]
1
35.5 =
[O2]
[O2] = 0.028 M
X =
0.056 mol = 2 X 0.028 =
١٣٤
135