Page 15 - E-LKM ACID BASE
P. 15
Lembar Kerja Mahasiswa - Asam Basa
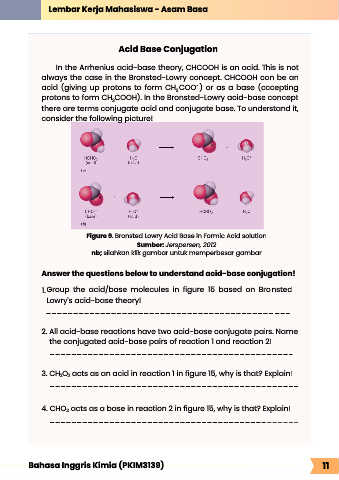
Acid Base Conjugation
In the Arrhenius acid-base theory, CHCOOH is an acid. This is not
always the case in the Bronsted-Lowry concept. CHCOOH can be an
-
acid (giving up protons to form CH COO ) or as a base (accepting
3
protons to form CH COOH). In the Bronsted-Lowry acid-base concept
3
there are terms conjugate acid and conjugate base. To understand it,
consider the following picture!
Figure 9. Bronsted Lowry Acid Base in Formic Acid solution
Sumber: Jerspersen, 2012
nb; silahkan klik gambar untuk memperbesar gambar
Answer the questions below to understand acid-base conjugation!
1. Group the acid/base molecules in figure 15 based on Bronsted
Lowry's acid-base theory!
_____________________________________________
2. All acid-base reactions have two acid-base conjugate pairs. Name
the conjugated acid-base pairs of reaction 1 and reaction 2!
_____________________________________________
3. CH O acts as an acid in reaction 1 in figure 15, why is that? Explain!
2 2
______________________________________________
4. CHO₂ acts as a base in reaction 2 in figure 15, why is that? Explain!
______________________________________________
Bahasa Inggris Kimia (PKIM3139) 11