Page 10 - E-LKM ACID BASE
P. 10
Lembar Kerja Mahasiswa - Asam Basa
-
+
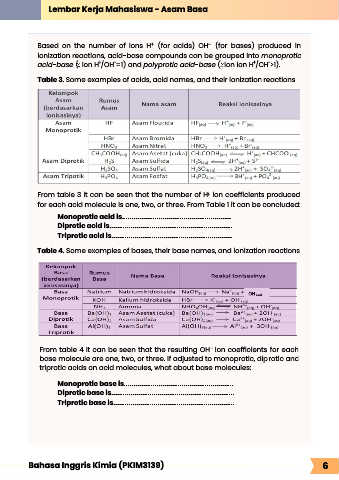
Based on the number of ions H (for acids) OH (for bases) produced in
ionization reactions, acid-base compounds can be grouped into monoprotic
+
-
-
+
acid-base ( ion H /OH =1) and polyprotic acid-base ( ion ion H /OH >1).
Table 3. Some examples of acids, acid names, and their ionization reactions
From table 3 it can be seen that the number of H ion coefficients produced
+
for each acid molecule is one, two, or three. From Table 1 it can be concluded:
Monoprotic acid is.........................................................
Diprotic acid is................................................................
Triprotic acid is...............................................................
Table 4. Some examples of bases, their base names, and ionization reactions
-
From table 4 it can be seen that the resulting OH ion coefficients for each
base molecule are one, two, or three. If adjusted to monoprotic, diprotic and
triprotic acids on acid molecules, what about base molecules:
Monoprotic base is.........................................................
Diprotic base is................................................................
Triprotic base is...............................................................
Bahasa Inggris Kimia (PKIM3139) 6