Page 14 - E-LKM ACID BASE
P. 14
Lembar Kerja Mahasiswa - Asam Basa
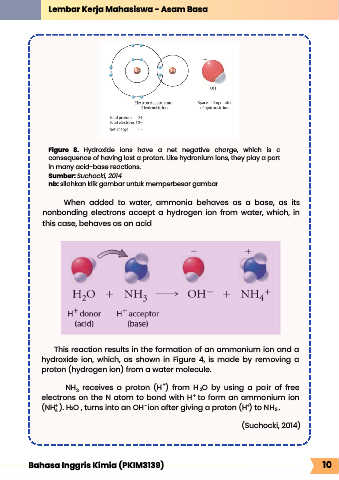
Figure 8. Hydroxide ions have a net negative charge, which is a
consequence of having lost a proton. Like hydronium ions, they play a part
in many acid-base reactions.
Sumber: Suchocki, 2014
nb: silahkan klik gambar untuk memperbesar gambar
When added to water, ammonia behaves as a base, as its
nonbonding electrons accept a hydrogen ion from water, which, in
this case, behaves as an acid
This reaction results in the formation of an ammonium ion and a
hydroxide ion, which, as shown in Figure 4, is made by removing a
proton (hydrogen ion) from a water molecule.
+
NH receives a proton (H ) from H O by using a pair of free
3
2
+
electrons on the N atom to bond with H to form an ammonium ion
+
-
+
(NH ). H O , turns into an OH ion after giving a proton (H ) to NH .
3
2
4
(Suchocki, 2014)
Bahasa Inggris Kimia (PKIM3139) 10