Page 480 - Veterinary Toxicology, Basic and Clinical Principles, 3rd Edition
P. 480
Manganese Chapter | 30 447
VetBooks.ir MECHANISM OF ACTION from the ETC (Turrens and Boveris, 1980), potentially
damage mitochondria directly or through the effects of
Mn is generally described as a neurotoxicant, selectively
secondary oxidants like superoxide, H 2 O 2 or peroxynitrite
affecting basal ganglia structures. Although it is known
2
(ONOO ), mediate Mn-induced oxidative damage.
that Mn is a cellular toxicant which can impair the trans-
Moreover, superoxide produced in the mitochondrial ETC
port system, enzyme activity and receptors function, the 21 31
may catalyze the transition shift of Mn to Mn
principal mechanism by which Mn neurotoxicity occurs
through a set of reactions similar to those mediated by
has not yet been clearly established (Aschner and
superoxide dismutase and thus lead to the increased
Aschner, 1991; Aschner et al., 2007; Martinez-Finley
oxidant capacity of this metal (Gunter et al., 2006).
et al., 2013; O’Neal and Zheng, 2015). Since mitochon-
Consequent oxidative damage produces an array of dele-
dria are the principal intracellular repository for metals
terious effects: it may cause structural and functional
(Cotzias and Greenough, 1958), binding of Mn to inner
derangement of the phospholipids bilayer of membranes,
mitochondrial membrane or matrix proteins (Gavin et al.,
disrupt energy metabolism, metabolite biosynthesis, cal-
1990) directly interacts with proteins involved in oxida-
cium and iron homeostasis and initiate apoptosis (Attardi
tive phosphorylation. Mn directly inhibits complex II
and Schatz, 1988; Yang et al., 1997; Uchida, 2003).
(Singh et al., 1974) and complexes I IV (Zhang et al.,
Consistent and preceding the Mn-induced increased in
2003) in brain mitochondria, and suppresses ATP-
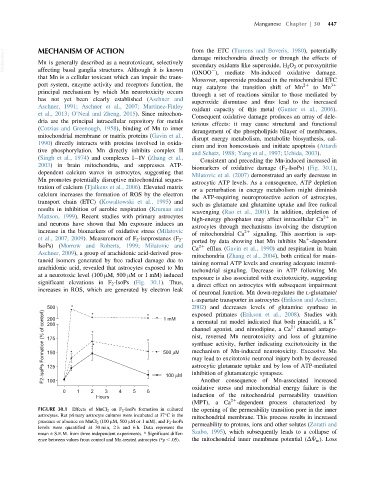
biomarkers of oxidative damage (F 2 -IsoPs) (Fig. 30.1),
dependent calcium waves in astrocytes, suggesting that
Milatovic et al. (2007) demonstrated an early decrease in
Mn promotes potentially disruptive mitochondrial seques-
astrocytic ATP levels. As a consequence, ATP depletion
tration of calcium (Tjalkens et al., 2006). Elevated matrix
or a perturbation in energy metabolism might diminish
calcium increases the formation of ROS by the electron
the ATP-requiring neuroprotective action of astrocytes,
transport chain (ETC) (Kowaltowski et al., 1995) and
such as glutamate and glutamine uptake and free radical
results in inhibition of aerobic respiration (Kruman and
scavenging (Rao et al., 2001). In addition, depletion of
Mattson, 1999). Recent studies with primary astrocytes 21
high-energy phosphates may affect intracellular Ca in
and neurons have shown that Mn exposure induces an
astrocytes through mechanisms involving the disruption
increase in the biomarkers of oxidative stress (Milatovic 21
of mitochondrial Ca signaling. This assertion is sup-
et al., 2007, 2009). Measurement of F 2 -isoprostanes (F 2 - 1
ported by data showing that Mn inhibits Na -dependent
IsoPs) (Morrow and Roberts, 1999; Milatovic and 21
Ca efflux (Gavin et al., 1990) and respiration in brain
Aschner, 2009), a group of arachidonic acid-derived pros-
mitochondria (Zhang et al., 2004), both critical for main-
tanoid isomers generated by free radical damage due to
taining normal ATP levels and ensuring adequate intermi-
arachidonic acid, revealed that astrocytes exposed to Mn
tochondrial signaling. Decrease in ATP following Mn
at a neurotoxic level (100 μM, 500 μM or 1 mM) induced
exposure is also associated with excitotoxicity, suggesting
significant elevations in F 2 -IsoPs (Fig. 30.1). Thus,
a direct effect on astrocytes with subsequent impairment
increases in ROS, which are generated by electron leak
of neuronal function. Mn down-regulates the L-glutamate/
L-aspartate transporter in astrocytes (Erikson and Aschner,
500 * * * 1 mM 2002) and decreases levels of glutamine synthase in
F2-IsoPs Formation (% of contorl) 175 * * * * 500 μM channel agonist, and nimodipine, a Ca channel antago-
exposed primates (Erikson et al., 2008). Studies with
200
1
a neonatal rat model indicated that both pinacidil, a K
200
21
nist, reversed Mn neurotoxicity and loss of glutamine
synthase activity, further indicating excitotoxicity in the
mechanism of Mn-induced neurotoxicity. Excessive Mn
150
may lead to excitotoxic neuronal injury both by decreased
astrocytic glutamate uptake and by loss of ATP-mediated
125
inhibition of glutamatergic synapses.
100 μM
Another consequence of Mn-associated increased
100
oxidative stress and mitochondrial energy failure is the
0 1 2 3 4 5 6
Hours induction of the mitochondrial permeability transition
21
(MPT), a Ca -dependent process characterized by
FIGURE 30.1 Effects of MnCl 2 on F 2 -IsoPs formation in cultured the opening of the permeability transition pore in the inner
astrocytes. Rat primary astrocyte cultures were incubated at 37 C in the mitochondrial membrane. This process results in increased
presence or absence on MnCl 2 (100 μM, 500 μM or 1 mM), and F 2 -IsoPs permeability to protons, ions and other solutes (Zoratti and
levels were quantified at 30 min, 2 h and 6 h. Data represent the
mean 6 S.E.M. from three independent experiments. * Significant differ- Szabo, 1995), which subsequently leads to a collapse of
ence between values from control and Mn-treated astrocytes (*p , .05). the mitochondrial inner membrane potential (ΔΨ m ). Loss