Page 84 - Veterinary Histology of Domestic Mammals and Birds, 5th Edition
P. 84
66 Veterinary Histology of Domestic Mammals and Birds
Connective tissue fibres are produced by the ribosomes of the rough endoplas-
VetBooks.ir COLLAGEN FIBRES (FIBRA COLLAGENOSA) mic reticulum. The resulting pro-α-chains are primarily
composed of glycine, proline and alanine, with non-helical
The scleroprotein collagen is the most commonly occur-
extensions (pro-peptides) at each end.
ring type of connective tissue fibre. It performs specific Within the endoplasmic reticulum, the proline and
protective and supportive functions in several tissues (e.g. lysine residues are hydroxylated and the pro-α-chains
skin, neuronal sheaths, tendons and ligaments, cartilage are joined to form triple (super) helices. Galactosyl
and bone). Collagen fibres also make up the interstitial and glucosyl residues are added in the Golgi appa-
connective tissue surrounding nerves and vessels, and ratus. The completed pro-collagen molecule is released
form the stroma that binds together the functional tissue from the Golgi apparatus within secretory vesicles that
(parenchyma) of various organs. pass along microtubules to the cell surface before being
Collagen fibres (Figures 3.7 and 3.10) have character- released by exocytosis into the extracellular space.
istic chemical and physical properties: they swell in acid This is followed by cleavage of the pro-peptides by the
environments, undergo partial digestion in gastric secre- enzyme pro-collagen peptidase. The shortened triple heli-
tions (pepsin) and dissolve completely in alkali. Under ces, termed collagen molecules (formerly tropocollagen),
polarised light, collagen fibres are uniaxially birefringent are approximately 280 nm in length. Collagen molecules
(anisotropic). This is attributable to their transverse band- aggregate by polymerisation to form collagen fibrils. In this
ing (Figure 3.10; see also below). Due to their molecular process, individual collagen molecules join end to end (with
structure, collagen fibres have a high tensile strength with a gap between consecutive molecules) to form a microfibril.
a maximum stretching capacity of only 5% (Table 3.1). Covalent cross-linkages are also formed between adjacent
The arrangement of collagen fibres varies with the microfibrils. It is thought that the formation of microfibrils
type of connective tissue. Particularly in loose connective is initiated by electrostatic attraction between neigh-
tissue, the fibres exhibit an undulating course. In dense bouring collagen molecules, which results in a staggered
irregular connective tissue such as fascia and aponeuroses, arrangement of the microfibrils (thus, also, staggering of
they are generally interwoven in a criss-crossing pattern. the gaps between collagen molecules). As a result, collagen
The collagen fibres of tendons are predominantly arranged fibrils exhibit a distinctive transverse banding pattern when
in parallel (Figures 3.20 and 3.21). viewed using electron microscopy. The cross-linking of adja-
Collagen fibres are synthesised by specific cells, includ- cent microfibrils contributes significantly to the mechanical
ing fibroblasts, chondroblasts and osteoblasts. The process strength of collagen fibres (Figure 3.10).
of collagen production includes an intra- and an extracel- Microfibrils (diameter 20–300 nm) combine to form
lular phase (Figures 3.5 and 3.6). collagen fibrils (diameter 0.2–0.5 μm). Aggregation and
In the intracellular phase, the polypeptide collagen cross-linking of fibrils give rise to collagen fibres (diam-
precursor pro-collagen is formed. Initially, structurally eter 1–20 μm). These fibres are unbranched and typically
similar chains of over 1000 amino acids (α - and α -chains) form bundles of varying size.
1 2
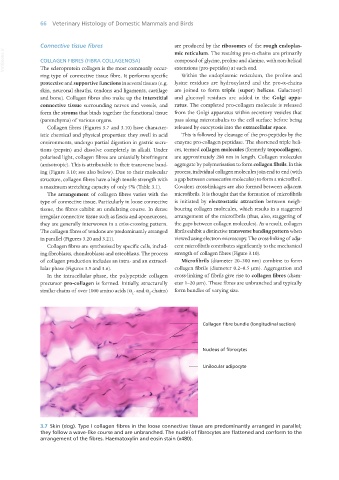
3.7 Skin (dog). Type I collagen fibres in the loose connective tissue are predominantly arranged in parallel;
they follow a wave-like course and are unbranched. The nuclei of fibrocytes are flattened and conform to the
arrangement of the fibres. Haematoxylin and eosin stain (x480).
Vet Histology.indb 66 16/07/2019 14:55