Page 181 - Medicinal Chemistry Self Assessment
P. 181
A
170 Medicinal Chemistry Self Assessment
B
C D
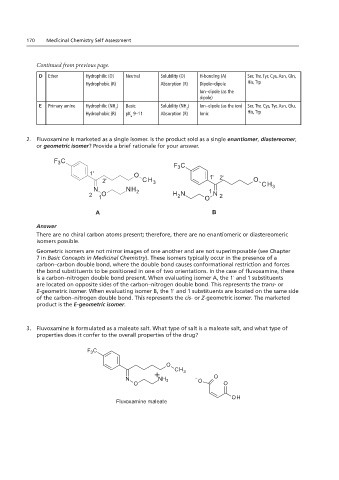
Continued from previous page.
D Ether Hydrophilic (O) Neutral Solubility (O) H-bonding (A) Ser, Thr, Tyr, Cys, Asn, Gln,
Hydrophobic (R) Absorption (R) Dipole–dipole His, Trp
E
Ion–dipole (as the
dipole)
Solubility (NH )
E Primary amine Hydrophilic (NH ) Basic Fluvoxamine Ion–dipole (as the ion) Ser, Thr, Cys, Tyr, Asn, Glu,
2 2
Hydrophobic (R) pK 9–11 Absorption (R) Ionic His, Trp
a
1. Conduct a to the questions that follow.
2.14 – remove bold from label
2. Fluvoxamine is marketed as a single isomer. Is the product sold as a single enantiomer, diastereomer,
2. Fluvoxamine is isomer? Provide a brief rationale for your answer.
or geometric isomer? Provide a brief rationale for your answer.
F C O O F C
3
3
1' O O OH
2' CH 3C CH 1' 2' O CH
H
Cl N NH 2 3 3 1 3
2 1 O H 2 N O N 2
A Fenofibric Acid B
Answer
1.15 and 2.15 – remove bold from label
There are no chiral carbon atoms present; therefore, there are no enantiomeric or diastereomeric
3. Fluvoxamine is properties of the drug?
isomers possible.
A
Geometric isomers are not mirror images of one another and are not superimposable (see Chapter
B
7 in Basic Concepts in Medicinal Chemistry). These isomers typically occur in the presence of a
carbon–carbon double bond, where the double bond causes conformational restriction and forces
D
C
the bond substituents to be positioned in one of two orientations. In the case of fluvoxamine, there
is a carbon–nitrogen double bond present. When evaluating isomer A, the 1' and 1 substituents
are located on opposite sides of the carbon–nitrogen double bond. This represents the trans- or
E-geometric isomer. When evaluating isomer B, the 1' and 1 substituents are located on the same side
E
of the carbon–nitrogen double bond. This represents the cis- or Z-geometric isomer. The marketed
product is the E-geometric isomer.
Fluvoxamine maleate
Fluvoxamine
3. Fluvoxamine is formulated as a maleate salt. What type of salt is a maleate salt, and what type of
properties does it confer to the overall properties of the drug?
1.15 and 2.15 – remove bold from label
F C
3
4. Fluvoxamine is well absorbed, provide a rationale for these pharmacokinetic properties.
O CH 3
N NH 3 – O O
O O
OH
Fluvoxamine maleate