Page 176 - Medicinal Chemistry Self Assessment
P. 176
Section 4 Whole Molecule Drug Evaluation
Answers
2.14 Fenofibrate and Gemfibrozil
Fenofibrate is a member of the fibrate class of anti-hyperlipidemic agents. It is used as adjunctive therapy to diet in the
management of dyslipidemias, including in the treatment of severe hypertriglyceridemia. The specific mechanism(s)
by which fenofibrate decreases triglyceride and total cholesterol levels, as well as increases the levels of high density
lipoproteins (HDL), is unknown. What we do know is that the decrease in very low density lipoproteins (VLDLs)
1.14 and 2.14 (drug name – remove bold)
results from fenofibrate stimulation of lipoprotein lipase.
Fenofibrate Gemfibrozil
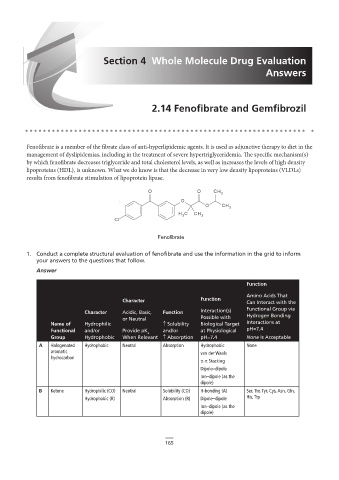
1. Conduct a complete structural evaluation of fenofibrate and use the information in the grid to inform
Letter “C” – add bold
your answers to the questions that follow.
Answer
Function
Amino Acids That
Character Function Can Interact with the
Character Acidic, Basic, Function Interaction(s) Functional Group via
Hydrogen Bonding
or Neutral Possible with
C
Name of Hydrophilic ↑ Solubility Biological Target Interactions at
Functional and/or Provide pK and/or at Physiological pH=7.4
a
Group Hydrophobic When Relevant ↑ Absorption pH=7.4 None Is Acceptable
A Halogenated Hydrophobic 2.14 – remove bold from label Hydrophobic None
Neutral
Absorption
aromatic van der Waals
hydrocarbon
π-π Stacking
B F
Dipole–dipole
A C D Ion–dipole (as the
dipole)
B Ketone Hydrophilic (CO) Neutral Solubility (CO) H-bonding (A) Ser, Thr, Tyr, Cys, Asn, Gln,
Hydrophobic (R) Absorption (R) Dipole–dipole His, Trp
E Ion–dipole (as the
dipole)
Fenofibrate
2.14 – remove bold from label
165
O O CH 3 O O
Ester
O Hydrolysis O
O CH 3 OH
H 3 C CH 3 H 3 C CH 3
Cl Cl
Inactive Active